- Wrought metal is obtained from cast metal. A wrought metal or alloy is one that has been worked, drawn, or shaped into a serviceable form, for example. plates, band materials, bars, and wires.
- The process of forming wrought metal objects has been known since ancient times.
- For example, swords used in warfare were formed by subjecting a hot piece of metal to a beating process.
- Other things used in daily life like farming equipment and kitchen utensils are also made by a similar process.
Read And Learn More: Basic Dental Materials Notes
Manufacture Of Wrought Alloys
- Wrought metal is usually derived from cast metal or alloy.
- Wrought metal is formed when the parent metal is subject to various deformative processes like drawing, extruding, machining, beating, rolling, forging, etc.
Examples of some of these processes are
- Round wires are obtained by drawing a cast alloy through a series of dies.
- The rolling process is used to form sheets and rods.
- Forging is a process by which an object is formed by compressing the parent metal between two dies. Stainless steel crowns are made by this process.
- The manufacture of wrought alloys results in a tremendous amount of stress (known as work hardening).
- These stresses are relieved by heat treatment during or after manufacture.
Structure Of Wrought Alloys
- All alloys are initially formed by casting. When cast metal is subject to any deformation,
- it is considered wrought metal. Wrought alloys have a fibrous structure which results from the cold working applied during the drawing operation to shape the wire.
- At the atomic level, the deformative processes involved in the manufacture of wrought alloys result in various types of atomic deformations and disruptions.
- These include dislocations, twinning, and fracture.
Wrought Alloys Dislocations
On application of a shear force, dislocation of the atoms occurs along a plane called as the slip plane. The simplest type of dislocation is known as edge dislocation. The dominant slip planes are characteristic of each type of crystal structure.
- For example, face-centered cubic (fcc) structures have the greatest number of slip planes.
- Therefore, metals with a fcc structure like gold, copper, nickel, palladium, silver, platinum, etc. are highly ductile and easy to draw.
- Body-centered cubic (bcc) metal have intermediate levels of ductility.
- hexagonal close-packed structures (hcp) have the least amount of slip systems and therefore are relatively brittle, for example, zinc.
- Dislocations occur only in materials having a crystalline structure.
- Dislocations cannot exist in materials with a noncrystalline structure like dental ceramics and polymeric materials.
Wrought Alloys Twinning
Another type of permanent deformation is known as twinning. The deformation occurs along either side of a plane in such a way that it mirrors each other. Twinning is favored over dislocation in metals that have relatively few slip systems.
Wrought Alloys Fracture
Continuation of cold working in a heavily deformed metal eventually leads to fracture. The fracture initiates from microcracks that occur at points where there is an accumulation of dislocations or at boundaries between different microstructural phases.
- Alloys can undergo brittle or ductile fracture depending on a variety of factors, such as composition, microstructure, and strain rate.
- When a ductile alloy fractures under tension, there is a reduction in the diameter of the metal (necking down) at the fracture site prior to fracture.
- Ductile fracture sites are characterized by a dimpled morphology. Microvoids or porosities may be seen at the fracture site.
- Fracture due to cold working is a cause for concern in dentistry. Examples are fractures of endodontic instruments like root canal files and reamers within the canal.
- Retrieval of such instruments can often be diffilt.
- That is why it is necessary to use these instruments in the correct sequence and manner and to change these instruments at regular intervals rather than use them till it breaks.
Annealing
The effects of cold working like strain hardening, susceptibility to corrosion, and loss of ductility can be neutralized by a heating process called annealing.
Stages of annealing
Annealing takes place in three stages
- Recovery
- Recrystallization
- Grain growth
The time and temperature for annealing is dependent on the melting temperature of the alloy. A commonly observed rule is to use a temperature that is approximately half the melting point of the metal or alloy on the absolute scale (K).
Recovery
- In the recovery stage, there is a slight decrease in tensile strength with no change in ductility.
- The most important beneficial changes occur during the recovery phase. As mentioned earlier, cold-worked metal contains a lot of residual stresses.
- The purpose of annealing heat treatment is to relieve these stresses. Maximum stress relief occurs during the recovery stage.
Recrystallization
- On further heating, changes in the microstructure begin to take place.
- The deformed grains begin to recrystallize forming new stress-free grains.
- The metal essentially regains its old soft and ductile condition.
- The metal loses its properties of resilience rendering it useless for its intended purpose.
- Thus recrystallization must be avoided.
Grain growth
- In this phase, the recrystallized grains continue to grow with larger grains consuming smaller grains.
- Grain growth does not proceed indefinitely, but rather ceases until a coarse grain structure is formed.
- There is no significant difference in ductility and tensile strength from that observed in the previous stage.
- Significance It is clear from the above that annealing should be done only until the recovery stage.
- Uncontrolled heating of dental-related appliances can result in unintended changes within the structure.
Uses Of Wrought Alloys
- Orthodontic wires
- Prosthodontic clasps
- Root canal instruments like fies and reamers
- Steel bands and brackets for orthodontic and pedodontics use
- Stainless steel crowns
- Dental instruments
Wrought Metals And Alloys Notes
Various types of wires are used in fixed and removable orthodontics for tooth movement and stabilization.
Classifiation (ISO 15841:2014)
Wires are classified on the basis of their elastic behavior.
- Type 1 wires: Wires displaying linear elastic behavior during unloading at temperatures up to 50 °C.
- Type 2 wires: Wires displaying nonlinear elastic behavior during unloading at temperatures up to 50 °C.
General Properties Of Orthodontic Wires
- Orthodontic wires are formed into various configurations or incorporated into appliances.
- When activated, these wires apply forces to the teeth and move them to the desired alignment.
- The force is determined by the appliance design and the material properties of the wire.
The following properties are important in orthodontic treatment.
- Force generated
- The force generated by the wire on the tooth is dependent on its composition and design.
- For a given design, the force generated is proportional to the wire’s stiffness.
- Elastic deflection and working range
- Biologically, low constant forces are less damaging.
- This is best achieved by a large elastic deflection because
- it produces a more constant force and has a greater ‘working range’.
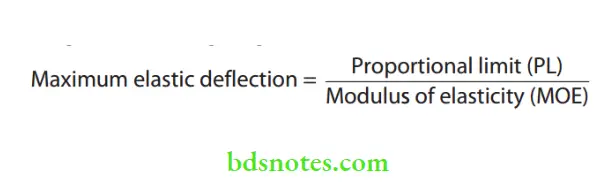
- Springiness It is a measure of how far a wire can be deflected without causing permanent deformation.
- Stiffness amount of force required to produce a specific deformation. Stiffness = 1/springiness
- Resilience It is the energy storage capacity of the wires which is a combination of strength and springiness.
- Formability It represents the amount of permanent bending; the wire will tolerate before it breaks.
- Ductility of the wire.
- Ease of joining Most wires can be soldered or welded together.
- Corrosion resistance and stability in the oral environment is important for the appliance’s durability as well as biocompatibility.
- Biocompatibility Most orthodontic wires are biocompatible. People generally allergic to nickel may get allergic reactions from nickel-containing orthodontic wires.
- Cost is a factor in orthodontics. The titanium alloy wires are more expensive than the stainless steel or the cobalt-chromium nickel wires.
Orthodontic Wires Types
- Wrought gold alloys
- Wrought base-metal alloys
- Stainless steel
- Cobalt-chromium-nickel
- Nickel-titanium
- Beta-titanium
Wrought Gold Alloys
Wrought Gold Alloys uses
Primarily to make clasps in partial dentures.
Wrought Gold Alloys classification
- Type 1—High precious metal alloys
- Type 2—Low precious metal alloys
Wrought Gold Alloys Composition
The composition varies widely.
- Gold — 25 to 70%
- Platinum — 5 to 50%
- Palladium — 5 to 44%
- Silver — 5 to 41%
- Copper — 7 to 18%
- Nickel — 1 to 3%
- Zinc — 1 to 2%
Wrought Gold Alloys properties
- They generally resemble Type IV casting gold alloys.
- Because of the cold working, wires and other wrought forms have improved mechanical properties like hardness and tensile strength
- when compared to cast structures.
- however, care should be taken during soldering.
- Prolonged heating at higher temperatures can cause it to recrystallize.
- Recrystallization changes the properties and makes the wire brittle.
Wrought Base-Metal Alloys
A number of wrought base-metal alloys are used in dentistry, mainly as wires for orthodontic treatment. The alloys are
- Stainless steel (iron-chromium-nickel)
- Cobalt-chromium-nickel
- Nickel-titanium
- Beta-titanium
Stainless steel
- Steel is an iron-based alloy which contains less than 1.2% carbon. When chromium (12 to 30%) is added to steel, the alloy is called as stainless steel.
- Elements other than iron, carbon, and chromium may also be present, resulting in a wide variation in the composition and properties of stainless steel.
Passivation
- Stainless steels are resistant to tarnish and corrosion, because of the passivating effct of the chromium.
- A thin, transparent but tough and impervious oxide layer forms on the surface of the alloy when it is exposed to air, which protects it against tarnish and corrosion.
- It loses its protection if the oxide layer is ruptured by mechanical or chemical factors.
Stainless steel types
There are three types of stainless steel based on the lattice arrangement of iron.
- Ferritic
- Martensitic
- Austenitic
- Duplex
- Precipitation hardening
Ferritic Stainless Steels
Pure iron at room temperature has a body-centered cubic (BCC) structure and is referred to as ferrite, which is stable up to 912 °C.
Properties and use
The ferric alloys have
- Good corrosion
- Resistance, but
- Less strength and
- Hardness.
So they find little application in dentistry.
Martensitic Stainless Steels
- When austenite (face-centered cubic structure) is cooled very rapidly (quenched), it will undergo a spontaneous, diffusionless transformation to a body-centered tetragonal (BCT) structure called martensite.
- This is a highly distorted and strained lattice, which results in a very hard and strong but brittle alloy.
Martensitic Stainless Steels Properties and uses
- The corrosion resistance of the martensitic stainless steel is less than that of the other types.
- Because of their high strength and hardness, martensitic stainless steels are used for surgical and cutting instruments.
- Bur shanks are also made from this steel.
Austenitic Stainless Steels
- At temperatures between 912 °C and 1394 °C, the stable form of iron is a face-centered cubic (FCC) structure called austenite.
- Austenitic stainless steel alloys are the most corrosion-resistant of stainless steels.
Austenite-Finish Temperature
It is the temperature at which the metallurgical transformation from the low-temperature martensite phase to the high-temperature austenite phase is completed.
Austenitic Stainless Steels Composition
- Chromium — 18%
- Nickel — 8%
- Carbon — 0.08-0.15%
Austenitic Stainless Steels uses
- This alloy is also known as 18-8 stainless steel.
- They are commonly used by orthodontists and pedodontists in the form of bands and wires.
- Type 316 L (contains carbon-0.03% maximum) is the type usually used for implants.


Available As
- They are available as annealed and partially annealed wires.
- They are usually supplied as rolls of varying thicknesses.
Austenitic Stainless Steels Advantages
Austenitic steel is preferred to ferritic alloys because of some desirable properties
- Greater ductility and ability to undergo more cold work without breaking.
- Substantial strengthening during cold working.
- Greater ease of welding.
- The ability to readily overcome sensitization.
- Less critical grain growth.
- Comparative ease in forming.
Austenitic Stainless Steels Properties
Sensitization
- The 18-8 stainless steel may lose its resistance to corrosion if it is heated between 400 and 900 °C (the temperature used during soldering and welding).
- The reason for a decrease in corrosion resistance is the precipitation of chromium carbide at the grain boundaries at these high temperatures.
- The small, rapidly diffusing carbon atoms migrate to the grain boundaries from all parts of the crystal to combine with the large, slowly diffusing chromium atoms at the periphery of the grain. When the chromium combines with the carbon in this manner, its passivating qualities are lost and the corrosion resistance of the steel is reduced.
Stabilization (methods to minimize sensitization)
- From a theoretical point, the carbon content of the steel can be reduced to such an extent that carbide precipitation cannot occur. however, this is not economically practical.
- By stabilization, i.e., some element is introduced that precipitates as a carbide in preference to chromium.
- Titanium is commonly used. Titanium at six times the carbon content, inhibits the precipitation of chromium carbide at soldering temperatures.
- These are known as stabilized stainless steel.
Annealed And Partially Annealed Wires
When stainless steel wires are fully annealed, they become soft and highly formable.
- When it is partially annealed, the yield strength is increased and formability decreased.
- Stainless steel is available in different grades depending on their yield strength.
- Both the fully annealed and partially annealed wires are used as orthodontic wires.
Mechanical properties
In orthodontic wires, strength and hardness may increase with a decrease in the diameter because of the amount of cold working in forming the wire.
- Tensile strength — 2100 MPa
- Yield strength — 1400 MPa
- Hardness — 600 Khn
Braided And Twisted Wires
- Very small diameter stainless steel wires (about 0.15 mm) can be braided or twisted together to form either round or rectangular-shaped (about 0.4 to 0.6 mm in cross-section) wires.
- These wires are available as straight lengths or as formed archwires in the form of 3 strands or in increasing numbers of strands.
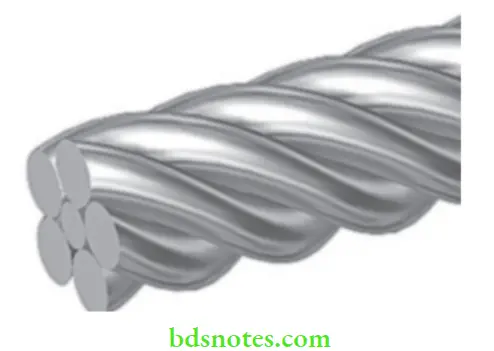
These braided or twisted wires are able to sustain large elastic deflections in bending and apply low forces for a given deflection when compared with solid stainless steel wire.
Soldiers For Stainless Steel
Silver solders are used. The soldering temperatures for orthodontic silver solders are in the range of 620 to 665 °C.
Fluxes
It is similar to that recommended for gold soldering with the exception of–
- The addition of the potassium fluoride. Fluoride helps to dissolve the passivating film supplied by the chromium.
- A higher boric acid to borax ratio lowers the fusion temperature.
Wrought Cobalt-Chromium-Nickel Alloys
These wrought alloys were originally developed for use as watch springs (Elgiloy). Their properties are excellent also for orthodontic purposes.
Cobalt-Chromium-Nickel Composition
- Co — 40%
- Cr — 20%
- Ni — 15%
- Mo — 7%
- Mn — 2%
- C — 0.15%
- Be — 0.04%
- Fe — 15.8%
Cobalt-Chromium-Nickel Heat Treatment
- Softening heat treatment 1100 to 1200 °C followed by a rapid quench. hardening heat treatment 260 to 650 °C, e.g. 482 °C for 5 hours.
- The wires are usually heat treated and supplied in several degrees of hardness (soft, ductile, semi-spring temper, and spring temper).
Cobalt-Chromium-Nickel Physical properties
- Tarnish and
- Corrosion
- Resistance is excellent.
- Hardness,
- Yield, and
- Tensile strength
- similar to those of 18-8 stainless steel.
Nickel-Titanium Alloys
Nickel-titanium shape memory alloys were first discovered by Buehler in the early 1960s.
- He was working at the Naval Ordinance Laboratory (nOL) at the time, hence the name nitinol.
- His discovery formed the basis of the first commercial shape memory alloy.
- These nickel-titanium alloy (also called nitinol) wires have large elastic deflections or working range and limited formability, because of their low stiffness and moderately high strength.
- They are used extensively as archwires in field orthodontic treatment.
- They are also used to manufacture endodontic instruments.

Nickel-TitaniuM AlloysAvailable As
- Nickel-titanium alloy wires are available as springs in addition to formed archwires.
- Nickel-titanium wires are commercially available in martensitic (M-niti) and austenitic (A-niti) depending on their use in different phases of orthodontic treatment.
Nickel-TitaniuM Alloys Composition
- The primary elements are nickel and titanium.
- The addition of copper to nickel and titanium alloy improves the thermal reactive properties of the wire, which help in consistent and efficient orthodontic tooth movement.
- Other additions made to alter the phase transformation temperature are elements such as iron and chromium which lower the temperature.
Properties Of Nitinol Alloys Shape Memory And Superelasticity
- This alloy exists in various crystallographic forms. At high temperatures, a stable body-centered cubic lattice (austenitic phase) exists.
- On appropriate cooling or an application of stress, this transforms to a close-packed hexagonal martensitic lattice with associated volumetric change.
- This behavior of the alloy (austenite to martensite phase transition) results in two features of clinical significance called as ‘shape memory’ and ‘superelasticity’, or ‘pseudoelasticity’.
- The ‘memory’ effct is achieved by first establishing a shape at temperatures near 482 °C.
- The appliance, for example, archwire is then cooled and formed into a second shape.
- Subsequent heating through a lower transition temperature (37 °C – mouth temperature) causes the wire to return to its original shape.
The phenomenon of superelasticity is produced by the transition of austenite to martensite by stress due to the volume change.
- which results from the change in the crystal structure. Stressing an alloy initially results in standard proportional stress-strain behavior.
- However, at the stress where it induces the phase transformation, there is an increase in strain, referred to as superelasticity.
- At the completion of the phase, it reverts to standard proportional stress-strain behavior. Unloading results in reverse transition and recovery.
- This characteristic is useful in some orthodontic situations because it results in low forces and a very large working range or spring back.
- These wires are useful because it is possible to achieve phase transformation at room temperature when force is applied.
Wires with different transformation temperatures are now available, which enables the clinician to select the precise wires for different needs.
- Density: Their density is approximately 6.5 g/cm3.
- Melting range: Melting temperature in the range of 1240 to 1310 °C.
Titanium Alloys
- Like stainless steel and nitinol, pure titanium has different crystallographic forms at high and low temperatures.
- At temperatures below 885°C the hexagonal close-packed (HCP) or alpha lattice is stable,
- whereas at higher temperatures the metal rearranges into a body-centered cubic (BCC) form called β-titanium.
- α-titanium is not used in orthodontic applications. Theβ-form is more useful in orthodontics.
- However, to retain theβ-form as it cools to room temperature elements like molybdenum are added.
- This stabilizes theβ-form and prevents its transformation to theα-form.
- For orthodontic use, the titanium alloys are supplied as precut arch wires usually in a rectangular cross-sectional form

Titanium Alloys Composition
- Ti — 79%
- Mo — 11%
- Zr — 6%
- Sn — 4%
Mechanical properties
- Modulus of elasticity – 70 GPa.
- Yield strength – 860 to 1200 MPa.
- The high ratio of yield strength to modulus produces orthodontic appliances that can undergo large elastic activations when compared with stainless steel.
- Beta-titanium can be highly cold-worked. It can be bent into various configurations and has formability comparable to that of austenitic steel.
- Welding Clinically satisfactory joints can be made by electrical resistance welding of beta-titanium.
- Corrosion resistance Both forms have excellent corrosion resistance and environmental stability.
- Heat treatment can alter its properties, therefore, heat treatment of these wires is not recommended.

Leave a Reply