Water
Question 1. Write short note on potable water.
Answer. Water used for human consumption should be both safe and wholesome and is known as potable water.
- Safe water is that water which does not harm the consumer even if it is ingested for long period.
- Wholesome water is the water which is agreeable to use without unpleasant taste or appearance.
- Potable water is physically, chemically and biologically suitable for drinking.
- Potable water is defined as water which should be free from pathogenic agents, free from harmful chemical substances, should be pleasant to taste and is useful for all domestic needs
- Hardness of potable water should not be above 150 mg of Caco/lt and its fluoride content should not be above 2 ppm.
Guidelines for Drinking Water Quality
The guidelines for drinking water quality recommended by WHO (1993 and 1996) relate to the following variables:
Acceptability Aspects
Following are the physical parameters:
- Drinking water should be free from turbidity. Water having turbidity of less than 5 nephelometric turbidity units (NPU) is acceptable.
- Drinking water should be free from color. The guideline value is up to 15 true color units.
- Drinking water should be free from taste and odor. There is no health based guideline value given for taste and odor.
Read And Learn More: Public Health Dentistry Question And Answers
Following are the inorganic constituents:
- Chlorides: Standard prescribed value for chloride is 200 mg/L. Its maximum permissible level is 600 mg/L. Presence of any excess chloride over the normal range is suspected of water contamination.
- Hardness: Drinking water should be moderately hard i.e. 1-3 mEq/L (50–150 mg/L).
- Ammonia: Its normal level in ground as well as surface water is below 0.2 mg/L. Anaerobic ground water may have below 0.2 mg/L. Presence of ammonia in water is indicative of possible bacterial sewage and animal waste pollution.
- pH: Acceptable pH for drinking water is between 6.5 and 8.5.
- Hydrogen sulphide: The test as well as odor threshold of hydrogen sulphide in water is estimated to be between 0.05 and 0.1 mg/L.
- Iron: Anaerobic groundwater can consists of ferrous ion at concentration up to several mg/L without causing any discoloration or turbidity in water. But when it is exposed to the atmosphere, the ferrous ion oxidizes to ferric ion, giving a reddish brown color to the water. Iron also causes growth of iron bacteria.
- Sodium: Taste threshold concentration of sodium in water always depends on the associated anion and the temperature of the solution. At room temperature, average taste threshold for sodium is about 200 mg/L.
- Sulphate: Presence of sulphate in the drinking water can produce noticeable taste. Taste impairment is minimum at levels below 250 mg/L.
- Total dissolved solids: Palatability of water with total dissolved solids (TDS) level less than 600 mg/L is considered to be good. Water having extremely low concentrations of TDS is unacceptable because of its flat, insipid taste. Drinking water becomes increasingly unpalatable at TDS levels greater than 1200 mg/L.
- Zinc: It produces an astringent taste to water which is undesirable. Its threshold concentration is 4 mg/L.
- Manganese: Concentration of manganese below 0.1 mg/L is acceptable. Levels above 0.1 mg/L, manganese leads to undesirable taste in beverages.
- Dissolved oxygen: Depletion of dissolved oxygen inside the water supplies encourages microbial reduction of nitrate to nitrite and sulphate to sulphide, which causes odor issue.
- Copper: Presence of copper above 1 mg/L can interfere with intended domestic uses of water.
- Aluminium: Its concentration should not exceed 0.2 mg/L.
Microbiological Aspects
Bacteriological Indicators
Drinking water should not have any pathogenic microorganisms.
It should be free from bacteria.
Bacterial indicators recommended for this purpose are:
- Coliform organisms: Coliform organism consists of both fecal and non-fecal microorganisms. Example for fecal group is E. coli and for non-fecal group is Klebsiella aerogens. Coliform organisms are seen in the human intestine. Coliform organisms are foreigners to the drinking water. Presence of coliform organisms in drinking water shows that water is contaminated by fecal matter.
- Fecal Streptococci: As name suggests this organism is seen in fecal matter. Its presence in water confirms the fecal contamination of water.
- Clostridium perfringens: It is present in fecal matter and its presence in water is indicative of fecal contamination occur at some remote time.
Virological Aspects
As per WHO it is recommended that drinking water should not contain any virus which is infectious to human.
Biological Aspects
- Protozoa: Potable water should not have any pathogenic intestinal protozoa.
- Helminths: Mature larva or a fertilized egg leads to infection such infections should not present in drinking water.
- Free living organisms: Free living organisms which occur in water are fungi and algae which provide bad odor and taste to drinking water.
Chemical Aspects
Presence of certain chemicals in excess of prescribed limits may make water non-potable.
Inorganic constituents:
- Arsenic: Provisional guideline for arsenic in drinking water is 0.01 mg/L.
- Cadmium: Guideline value for cadmium is established at 0.003 mg/L.
- Chromium: Guideline value for chromium is 0.05 mg/L.
- Cyanide: Guideline value of 0.07 mg/L is considered to be safe.
- Fluoride: Guideline value suggested is l.5 mg/litre
- Lead: Health based guideline value of lead is 0.01 mg/L
- Mercury: Guideline value for total mercury is 0 00l mg/L.
- Nitrate and nitrite: Guideline value of 3 mg/L for nitrite and 50 mg/L for nitrate has been proposed.
- Selenium: The guideline value is 0.01 mg/L
Organic Constituents:
- Polynuclear aromatic hydrocarbons: In situations where there is contamination of drinking water by polynuclear aromatic hydrocarbons has occurred, the specific compounds present and the source of the contamination should be identified, as the carcinogenic potential of polynuclear aromatic hydrocarbon compounds varies.
- Pesticides: It consists of chlorinated hydrocarbons and their derivatives. For example, DDT concentration in water should not exceed 2 mg/L.
Radiological Aspects
- Effects of radiation exposure are known as somatic if they become manifest inside the exposed individual and hereditary if they affect the descendants.
- Mainly the activity of a radioactive material is the number of nuclear disintegration per unit of time. The unit of activity is a becquerel (Bq).
- 1 Bq = 1 disintegration per second.
- Proposed guideline values are as follows:
- Gross alpha activity 0.1 Bq/L
- Gross beta activity 1.0 Bq/L.
Question 2. Write short note on guidelines for drinking water quality recommended by WHO, its microbiological aspect.
Answer. Following are the guidelines for drinking water quality recommended by WHO (1993 and 1996), its microbiological aspects:
Bacteriological Indicators
Drinking water should not have any pathogenic microorganisms.
It should be free from bacteria.
Bacterial indicators recommended for this purpose are:
- Coliform organisms: Coliform organism consists of both fecal and nonfecal microorganisms. Example for fecal group is E. coli and for nonfecal group is Klebsiella aerogens. Coliform organisms are seen in the human intestine. Coliform organisms are foreigners to the drinking water. Presence of coliform organisms in drinking water shows that water is contaminated by fecal matter.
- Fecal Streptococci: As name suggests this organism is seen in fecal matter. Its presence in water confirms the fecal contamination of water.
- Clostridium perfringens: It is present in fecal matter and its presence in water is indicative of fecal contamination occur at some remote time.
Virological Aspects
As per WHO it is recommended that drinking water should not contain any virus which is infectious to human.
Biological Aspects
- Protozoa: Potable water should not have any pathogenic intestinal protozoa.
- Helminths: Mature larva or a fertilized egg leads to infection such infections should not present in drinking water.
- Free living organisms: Free living organisms which occur in water are fungi and algae which provide bad odor and taste to drinking water.
Question 3. Write short note on purification of water.
Or
Write short note on water purification methods.
Answer. Purification of water is of great importance in community medicine.
It may be considered under two headings:
- Purification of water on a large scale
- Purification of water on a small scale.
Purification of Water on a Large Scale
Components of water purification system consist of storage, filtration and chlorination.
Storage:
This is a natural purification.
As a result of storage, a very considerable amount of purification takes place.
- Physical: Quality of water improves because suspended impurities settle down.
- Chemical: Aerobic bacteria oxidize the organic matter present in the water with the help of dissolved oxygen.
- Biological: A tremendous drop takes place in bacterial count during storage. The pathological organisms die out.
Filtration:
Two types of filtration are used:
- Biological or slow sand filtration
- Mechanical or rapid sand filtration.
Slow Sand Filtration Consist of
- Supernatant water
- A bed of graded sand
- An under drainage system
- A system of filter control values.
Rapid Sand or Mechanical Filters
Two types:
- Gravity type
- Pressure type
- Steps involved in the purification of water by rapid sand filter:
- Coagulation
- Rapid mixing
- Flocculation
- Sedimentation
- Filtration
- Steps involved in the purification of water by rapid sand filter:
Chlorination:
Chlorination is a supplement to sand filtration; chlorine kills the pathogenic bacteria, but it has no effect on spores and certain viruses.
Action of Chloride
- When chloride is added to water there is formation of HCl and hypochlorous acid.
- Disinfecting action of chloride is mainly due to hypochlorous acid, and to small extent due to hypochloride ions.
Methods of Chlorination
- Chlorine gas: It is cheap, quick in action, efficient and easy to apply.
- Chloramine: Loose compounds of chlorine and ammonia. They have fewer tendencies to produce chlorinous taste, slow in action.
- Perchloron: Calcium compound, which carries 60 to 70 percent of chlorine.
Other Agents
- Ozonation
- Ultraviolet radiation.
Purification of Water on a Small Scale
- Household purification of water:
- Boiling
- Chemical disinfection
- Bleaching powder
- Chlorine solution
- Chloride tablet
- High test hypochlorite
- Iodine
- Potassium permanganate.
- Filtration
- Ultraviolet irradiation
- Multistage reverse osmosis purification of water
- Disinfection of wells.
Question 4. Define potable water. Write in detail about purification of water on large scale.
Answer. Potable water is defined as water which is:
- Free from pathogenic agents
- Free from harmful chemical substances
- Pleasant to the taste, i.e. free from color and odor
- Useful for all domestic needs.
Purification of Water on Large Scale
Water purification system consists of three components, i.e.
- Storage
- Filtration
- Chlorination
Storage
At the time of storage, considerable purification occur by
- Physical action: 90% of the suspended impurities settle down under 24 hours by action of gravity. Water get clearer allowing the penetration of light.
- Chemical action: Here, aerobic bacteria oxidize the organic matter which is present in the water with the help of dissolved oxygen which reduces content of free ammonia and increases the concentration of nitrates.
- Biological action: During storage pathogenic organisms slowly die out. But if the water is stored for long periods, there are chances of development of vegetable growths i.e. algae, which imparts bad smell and color to the water.
Filtration
It is the second stage in purification of water.
Here two types of filters are used i.e.,
- The biological or “slow sand” filters
- The rapid sand or mechanical filters
Slow sand or Biological filters
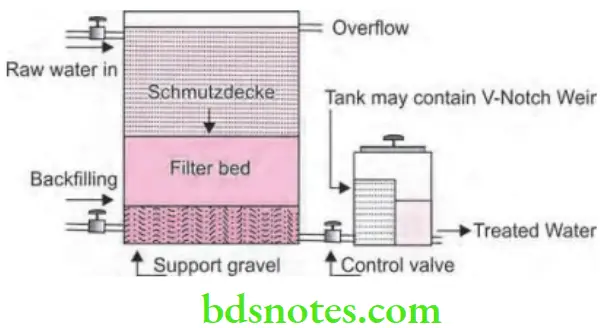
Elements of a slow sand filter are:
- Supernatant (raw) water: Depth of supernatant water above the sand bed varies from 1 to 1.5 meters. This also provides a constant head of water to overcome resistance of the filter bed and promote the downward flow of water into the sand bed. Due to this there is also the waiting period of some hours for the raw water to undergo purification by the sedimentation, oxidation and particle agglomeration.
- Bed of graded sand: Thickness of sand bed is 1 meter. The sand grains consists of an effective diameter between 0.2 and 0.3 mm. Sand bed is supported by a layer of graded gravel which is 30 to 40 cm deep and this also prevents fine grains being carried into the drainage pipes. Water percolates via the sand bed very slowly and is subjected to mechanical straining, sedimentation, adsorption, oxidation and bacterial action. Rate of filtration of water lies between 0.1 and 0.4 m3/hour/square meter of the surface of sand bed.
Slimy growth covering the surface of the sand bed is known as “Schmutzdecke”, vital layer, zoogleal layer or biological layer. It has threadlike algae, plankton, diatoms and bacteria. This may take some days for vital layer to form fully, as it is fully formed it extends 2 to 3 cm into the top most portion of sand bed. The formation of vital layer is known as “ripening” of the filter. Vital layer is the “heart” of the slow sand filter. This layer removes the organic matter, holds back bacteria and oxidizes ammoniacal nitrogen into nitrates and this is helpful for purifying the water. Till vital layer is fully formed, the first few days filtrate is usually run to waste. - An under drainage system: At the bottom of filter bed there is under drainage system which consists of the perforated pipes which provides outlet for the filter water and also supports filter medium above.
- A system of filter control values: Filter control values maintain a constant rate of filtration. Venturi meter is used to measure the bed resistance or loss of head. As resistance builds up, operator opens the regulating valve to maintain a steady rate of filtration.
Cleaning of Filter
As the bed resistance increases to the level that the regulating valve should be kept fully open, now it is time to clean the filter bed. Now supernatant water is drained off and cleaning of sand bed is done by scraping off the top portion of sand layer to a depth of 1 to 2 cm. After 20 or 30 scrapings, thickness of the sand bed will have reduced and a new bed is constructed.
Rapid Sand or Mechanical Filters
Here various steps involved are as follows.
- Coagulation: Raw water is treated with a chemical coagulum i.e. alum.
- Rapid mixing: Treated water is now subjected to violent agitation inside the “mixing chamber” for few minutes. This allows a quick and through dissemination of alum throughout the bulk of water:
- Flocculation: It involves slow and gentle stirring of treated water inside a flocculation chamber for half an hour. This leads to formation of a thick, copious, white flocculent precipitate of aluminum hydroxide.
- Sedimentation: Now the coagulated water led into sedimentation tank; where it should be kept for 2 to 6 hours, when the flocculent precipitate together along with impurities and bacteria settle down inside the tank.
- Filtration: Partly purified water is now subjected to rapid sand filtration.
Filter Beds
- Each of the unit of filter bed consists of surface of 80 to 90 m2.
- Sand is the filtering medium.
- Effective size of sand particles is between 0.4 to 0.7 mm.
- Depth of sand bed is usually about 1 meter.
- Below the sand bed there is a layer of graded gravel which is usually 30 to 40 cm deep.
- Gravel supports the sand bed and permits the filtered water to move freely towards the under drains. Depth of water on top of the sand bed is 1.0 to 1.5 m.
- The rate of filtration is 5 to15 m3/m2/hour.
Filtration eliminates the remaining alum floc which is not removed by sedimentation. As if the filtration proceeds, suspended impurities and bacteria clog the filters which result in their reduced efficiency known as loss of head.
Back Washing
Rapid sand filters has the demand of frequent washing either daily or weekly which depends on the loss of head. Washing is accomplished by reversing the flow of water through sand bed, this is known as back washing. It dislodges the impurities and cleans the sand bed. It should be stopped when the wash water is sufficiently clean. Whole process of washing takes 15 minutes.
Chlorination
Chlorination is a supplement but it is not considered as the substitute to sand filtration.
Actions of chlorine are as follows:
- Chorine is lethal to pathogenic bacteria, but it has no effect on spores and certain viruses except in high doses.
- Chlorine oxidizes iron, manganese and hydrogen sulphide.
- Chlorine destroys taste as well as odor producing constituents.
- Chlorine controls algae and slime organisms.
- Chlorine aids in coagulation.
Action of Chlorine:
As chlorine is added in the water there is formation of hydrochloric acid and hypochlorous acid. Hydrochloric acid is neutralized by alkalinity of water. Hypochlorous acid ionizes to form hydrogen ions and hypochlorite ions. Main disinfecting action of chlorine is due to hypochlorous acid.
Principles of Chlorination
- Water which is to be chlorinated should be clear and free from turbidity.
- Chlorine demand of water should be estimated. Chlorine demand of water is the difference between the amount of chlorine added to water and the amount of residual chlorine remaining at end of a specific period of contact at a given temperature and pH of water.
- Point at which the chlorine demand of water is met is known as break point. If further chlorine is added beyond the break point, free chlorine begins to appear inside the water.
- Free residual chlorine is present for a contact period of at least one hour to kill both bacteria and viruses.
- Minimum recommended concentration of free chlorine is 0.5 mg/L for one hour. This provides a margin of safety against subsequent microbial contamination which can occur during storage and distribution.
- Sum of chlorine demand of the water in addition with the free residual chlorine of 0.5 mg/L constitutes the correct dose of chlorine to be applied.
Method used to Apply Chlorine
- Chlorine gas: Chlorine applied as chlorine gas is the first choice since it is economical, quick in action, efficient and is easy to apply. Moreover it is an irritant to eyes and is poisonous.
- Chloramines: They are loose compounds of both ammonia and chlorine. They have less tendency to produce chlorinous taste and gives a more persistent type of residual chlorine.
- Perchloron: It is also known as high test hypochlorite and is a calcium compound.
Superchlorination
Superchlorination consists of addition of large doses of chlorine to water and removal of excess of chlorine by the dechlorination. This method is implicated for the heavily polluted river water.
Other agents used for water purification
- Ozonation: Ozone acts as a powerful oxidizing agent. It has a strong virucidal effect. It eliminates undesirable odor; taste and color. Its main disadvantage is that there is no residual germicidal effect.
- Ultraviolet irradiation: This is effective against most microorganisms including viruses. The apparatus which produces ultraviolet irradiation is expensive.
Question 5. What do you mean by ‘potable water’ and ‘Wholesome water’ How do you purify water at large scale.
Answer. Potable water is defined as water which is:
- Free from pathogenic agents
- Free from harmful chemical substances
- Pleasant to the taste i.e. free from color and odor
- Useful for all domestic needs.
Wholesome water is the water which is agreeable to use without unpleasant taste or appearance.
Question 6. Write in detail about slow sand filtration and add a note on chlorination of water.
Answer.
Slow Sand Filtration
- Slow sand filtration is a simple process.
- It is relatively inexpensive to build, but do require highly skilled operators.
- The process percolates untreated water slowly through a bed of porous sand, with the influent water introduced over the surface of the filter, and then drained from the bottom.
- Properly constructed, the filter consists of a tank, a bed of fine sand, a layer of gravel to support the sand, a system of under drains to collect the filtered water, and a flow regulator to control the filtration rate.
- No chemicals are added to aid the filtration process.
Elements of a Slow Sand Filter
- Supernatant water: The depth of the supernatant water above sand bed is 1–1.5 meters. Slow sand filter usually provides a constant head of water to overcome the resistance of filter bed and it usually prevents the downward flow of water into the sand bed. It generally provides waiting period of few hours for raw water to undergo purification by sedimentation, oxidation, and particle agglomeration.
- A bed of graded sand: The thickness of sand bed is approximately 1 meter. The effective diameter of sand grain should be 0.20 to 0.30 mm. The sand bed is supported by layer of graded gravel, 30 to 40 cm deep. This prevents the fine grains being carried into the drainage pipes. The newly laid filter soon gets covered with a slimy growth. This layer is called as “Schmutzdecke’, vital or biological layer. It removes the organic bacteria and holds back bacteria. It oxidizes ammoniacal nitrogen into nitrates and helps to yield bacteria free water.
- An under drainage system: It consists of perforated pipes through which filtered water is collected and it supports the filter medium above.
- A system of filter control valves: The outlet pipe system is equipped with valves, which helps to maintain a constant rate of filtration.
Cleaning of Filter
When the bed resistance get increased to so much extent that regulating valve should be kept open fully then the filter bed should be cleaned. The supernatant water is then drained and sand bed is cleaned by scrapping top portion of sand layer to depth of 1–2 cm. After maximum of 20-30 scrapings thickness of sand bed is reduced and new bed get constructed.
Cleaning of Filter Advantages
- Design and operation simplicity as well as minimal power and chemical requirements make the slow sand filter an appropriate technique for removing suspended organic and inorganic matter.
- These filters remove pathogenic organisms.
- Slow sand filtration reduces bacteria, cloudiness, and organic levels thus reducing the need for disinfection and, consequently, the presence of disinfection byproducts in the finished water.
- Sludge handling problems are minimal.
- Close operator supervision is not necessary.
- Systems can make use of locally available materials and labor.
Slow sand filtration Limitations
- They require a large land area
- Large quantities of filter media and manual labor for cleaning.
- Water with high turbidity levels can quickly clog the fine sand in these filters.
- Slow sand filters do not completely remove all organic chemicals, dissolved inorganic substances, such as heavy metals, or trihalomethane precursors chemical compounds that may form trihalomethane precursors when mixed with chlorine.
Chlorination of Water
- Chlorination is one of the greatest advances in water purification.
- Chlorine kills pathogenic bacteria, but it has no effect on spores and certain viruses except in high doses.
- It has limited effectiveness against protozoans that form cysts in water.
Mechanism of Action
- When chlorine is added to water there is formation of hydrochloric and hypochlorous acids.
- The hydrochloric acid is neutralized by the alkalinity of water. The hypochlorous acid ionizes to form hydrogen ions and hypochlorite ions.
- The disinfecting action of chlorine is mainly due to the hypochlorous acid and to a small extent due to the hypochlorite ions.
- Chlorine acts best as a disinfectant when the pH of water is around seven because about 90 % of the hypochlorous acid gets ionized to hypochlorite ions.
Method of Chlorination
Chlorine is applied as:
- Chlorine gas
- Chloramines
- Perchloron.
- Chlorine gas: It is the first choice as its action is fast, economical, efficient and is applied easily. It is a toxic gas, hence there is a danger of a release associated with its use.
- Chloramines: They are chlorine-based disinfectants. They are loose compounds of chlorine and ammonia.
- Perchloron: It is also known as hypochlorite.
Forms of Chlorination
- Plain chlorination: When raw water is supplied to consumer by applying chlorine treatment only.
- Pre-chlorination: When raw water is suspected to be highly contaminated, then a dose of chlorine is added to the raw water before it enters the sedimentation chamber.
- Post-chlorination: When chlorine is added to water after all the treatment is over, just before it enters the distribution system to prevent contamination in the distribution line.
- Double chlorination: When Pre and post-chlorination are both adopted.
- Break point chlorination: The point at which the residual chlorine appears and when all combined chlorines have been completely destroyed is the breakpoint and corresponding dosage is the breakpoint dosage.
- Superchlorination: It is followed by dechlorination and comprises the addition of large doses of chlorine to the water, and removal of excess of chlorine after disinfection, this method is applicable to heavily polluted water whose quality fluctuates greatly.
Question 7. Write short note on rapid sand filtration.
Answer. During rapid sand filtration the rapid sand filters are used.
Rapid sand filters are also known as mechanical filters.
Following steps are involved in this process:
- Coagulation: Raw water at first is treated with a chemical coagulant such as alum.
- Rapid mixing: The treated water is subjected to violent agitation in “mixing chamber” for few minutes. This allows a quick and thorough dissemination of alum throughout the bulk of water.
- Flocculation: It generally involves a slow and gentle stirring of treated water in flocculation chamber for about 30 minutes. This slow and gentle stirring results in the formation of a thick, copious, white flocculent precipitate of aluminium hydroxide.
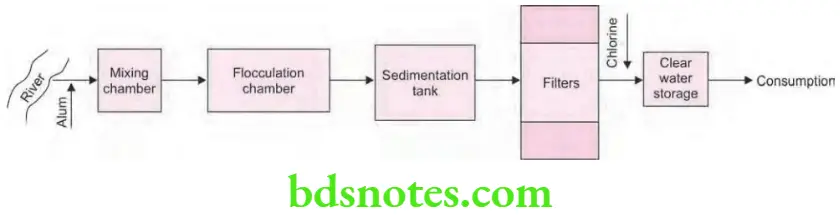
- Sedimentation: The coagulated water is now led into sedimentation tanks where it is detained for periods varying from 2 to 6 hours when the flocculent precipitate together with impurities and bacteria settle down in the tank.
- Filtration: Partly purified water is than subjected to rapid sand filtration.
Question 8. Write in brief differences between slow sand filter and rapid sand filter.
Answer.
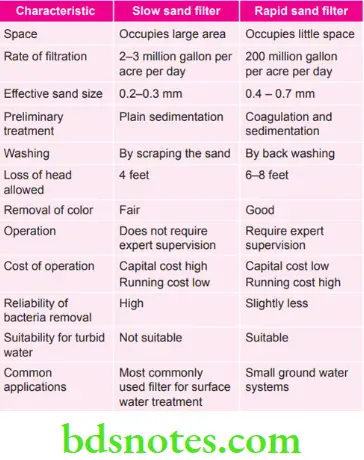
Question 9. Write short note on purification of water on a small scale.
Answer. Purification of water on a small scale is done by following methods:
- House-hold purification of water:
- Boiling
- Chemical disinfection
- Bleaching powder
- Chlorine solution
- Chloride tablet
- High test hypochlorite
- Iodine
- Potassium permanganate.
- Filtration
- Ultraviolet irradiation
- Multistage reverse osmosis purification of water
- Disinfection of wells.
Boiling
- Boiling is the best method of purification of water at household level. For its proper effect water is brought up to a rolling boil for 5 to 10 minutes.
- It kill all bacteria, spore and cysts.
- If boiling is done for 10-20 minutes viruses like hepatitis A get killed.
- By this method removal of temporary hardness of water occur.
- By use of this method there is no “residual protection”.
Chemical Disinfection
- Bleaching powder consists of 33% of available chlorine. Turbid water should be purified by this method
- Chlorine solution is used for water wells
- Chlorine tablets are used for travellers and in cases of emergencies.
- High test hypochlorite or perchloron contain 60 to 70% of chlorine which disinfect the water.
- Iodine is used for emergency purification of water. Its contact time is 20 to 30 minutes and is needed for effective disinfection.
- Potassium permanganate can kill cholera vibrio. It alters the color, smell and taste of water. So it is not used nowadays.
Filtration
- Water is purified on a small scale by filters i.e. Pasteur Chamberland filter, Berkefeld filter and “Katadyn” filter.
- Candle filters are used.
- Candle is made up of porcelain in chamberland type, kieselgurh in Berkefeld type and the surface of filter is coated with silver catalyst in Katadyn type.
- These candles remove bacteria found in the drinking water.
Ultraviolet Irradiation
- Ultraviolet irradiation is effective against most of organisms which contaminate water such as bacteria, yeast, fungi, algae, protozoa.
- In this the film of water is exposed to 120 mm thickness to one or several quartz mercury vapor arc lamps which emit ultraviolet radiation of 254 nm.
- By this method the water is effectively purified and there is no change in taste or odor and no foreign matter is introduced to water.
Multistage Reverse Osmosis Purification of Water
This method make water chemically and microbiologically more potable by reducing dissolve salts, hardness, heavy metals and microorganisms such as bacteria, viruses, protozoa, etc.
Disinfection of Wells
This is established by adding bleaching powder and chlorine.
Double Pot method is used by national environment engineering research institute (NEERI), Nagpur, India.
Following are the steps in disinfection of wells:
- Volume of water should be founded in the well.
- Amount of bleaching powder should be found which is required for disinfection.
- Bleaching powder should be dissolved in water.
- Chlorine solution should be dissolved in the well.
- Sufficient contact period should be allowed
- Residual chlorine level is measured.
Question 10. Write short note on purification of water on domestic level.
Answer. Following are the methods which are used for the purification of water on domestic level:
- Boiling
- Chemical disinfection
- Bleaching powder
- Chlorine solution
- Chloride tablet
- High test hypochlorite
- Iodine
- Potassium permanganate.
- Filtration
- Ultraviolet irradiation
- Multistage reverse osmosis purification of water.
Question 11. Write short note on break point chlorination
Answer. The addition of chlorine to ammonia in water leads to the formation of chloramines, which do not have the same efficiency as free chlorine.
- If the dose of chlorine in water is increased, there is a reduction in residual chlorine due to destruction of chloramines by added chlorine. The end products do not represent any residual chlorine.
- This fall in residual chlorine will continue with further increase in chlorine dosage and after a stage, the residual chlorine increase in proportion to the added dose of chlorine.
- The point at which the residual chlorine appears and when all combined chlorines have been completely destroyed is the breakpoint and corresponding dosage is the breakpoint dosage.
Question 12. Write short note on vital layer.
Answer. Vital layer refers to the slimy growth which covers the surface of sand bed.
- It is also known as Schmutzdecke or Zoogleal layer or biological layer.
- Vital layer consists of thread like algae, plankton, diatom and bacteria.
- Vital layer takes some days to complete its formation.
- When vital layer is formed completely, it extends 2 to 3 cm in the top portion of the sand bed.
- Formation of vital layer is known as “ripening” of the filter.
- Vital layer is basically the heart of slow sand filter.
- Function of vital layer is to remove organic matter, holds bacteria and oxidizes ammonical nitrogen into nitrates, this helps in purifying water.
- Till the vital layer is fully formed, the first few days filtrate is run to waste.

Leave a Reply