Translation Protein Synthesis
DNA, with its correct mechanism of replication, serves to carry genetic information from cell to cell and from generation to generation.
- This information is translated into proteins that determine the phenotype. Virtually, all the phenotypes examined so far are the result of biochemical reactions that occur in the cell. All of these reactions require enzymes and enzymes are proteins.
- More than 2000 types of enzymes have been identified in the living organisms. Each enzyme is a unique molecule catalyzing a specific chemical reaction.
- Other phenotypes are due primarily to the kinds and amounts of non-enzymatic proteins (including the structural proteins) present, for example, hemoglobin, myoglobin, gamma-globulin (for example., immunoglobulins or Igs), insulin, cytochrome C, fibroin (silk protein), or collagen.
- Proteins are composed of one or more, long linear polymers of amino acid residues (polypeptide chains) that are synthesized almost exclusively in the cytoplasm. The topic of this chapter is how the information present in the sequence of bases (=triplet codons) of the mRNA is translated into a sequence of amino acids in proteins.
- However, at the outset, one point should be clear that the genetic information of mRNA is undirectionally read beginning at the 5-hydroxyl end by one or more ribosomes (polysomes), and the 3′ – end signifies the C-terminal amino acid.
- The 5′ – end of the mRNA corresponds to the N-terminal amino acid and ends at the 3′-end in the completed protein.
Central Dogma And Central Dogma Reverse
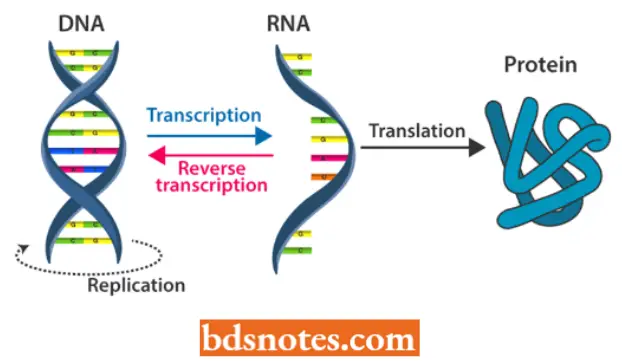
The process of synthesis of protein involves one of the central dogma of molecular biology, which postulates that genetic information (lows from nucleic acids to protein, (It was first forwarded by Crick in 195S).
- The first step of this central dogma is known as transcription and does not involve a change of code since DNA and mRNA are complementary.
- The second step involves a change of code from nucleotide sequences to amino acid sequences and is called translation. It can be illustrated as follows:

- Thus, according to this central dogma, the flow of information is one way, i.e., from DNA the information is transferred to RNA (mRNA) and from RNA to proteins. In 1968.
- Barry Commoner suggested a circular flow of information, i.e., DNA transcribes RNA, RNA translates into proteins, proteins synthesize RNA and RNA synthesizes DNA, as has been illustrated in the following:
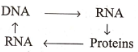 Later on, Temin (1970) reported the existence of an enzyme “RNA-dependent DNA polymerase” (reverse transcriptase) which could synthesize DNA from a single-stranded RNA template.
Later on, Temin (1970) reported the existence of an enzyme “RNA-dependent DNA polymerase” (reverse transcriptase) which could synthesize DNA from a single-stranded RNA template.
- D. Baltimore (1970) also reported the activity of this enzyme in certain tumor viruses.
- This exciting finding in molecular biology gave rise to the concept of “central dogma reverse” or feminism, suggesting that the sequence of information Ilow is not necessarily from DNA to RNA to protein but can also take place from RNA to DNA.
- The central dogma reverse can be illustrated as follows:
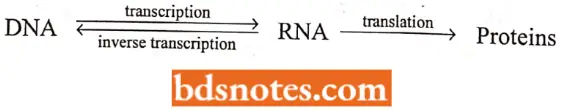
- The step of translation of this central dogma is the time in the flow of information between genes and proteins to change the language being used.
- In going from DNA to RNA, the language (nucleotide sequences) remained the same.
- However, in going from RNA to protein the language is changed from a nucleotide sequence to an amino acid sequence.
- Just as in the process of translation from one language to another, this process of using information in RNA to make protein is called translation.
Minimum Necessary Materials For Translation
Success in polypeptide synthesis in vitro cell-free systems shows that the minimum necessary materials for translation are the following:
- Amino acids (i.e., 20 amino acids forming the pool of amino acids in the cytoplasm)
- Ribosomes (each of which comprises two subunits that exist as separate subunits before the translation of mRNA contains two tRNA binding sites: the P site or peptidyl site and an A or aminoacyl site.
- One more site called E or exit site has been recognized in the ribosomes by some workers but this three-site model (i.e., A, P, and E) still is not popular.
- mRNA
- tRNA of several kinds
- Enzymes
- Amino-acid activating system ((for example., aminoacyl-tRNA-synthetase).
- Peptide polymerase system
- Adenosine triphosphate (ATP) as an energy source
- Guanosine triphosphate (GTP) for synthesis of peptide bonds
- Soluble protein for initiation and transfer factors
- Various inorganic cations (for example., K+, NH4+, Mg2+).
Because proteins are the polymers of amino acids, therefore, protein synthetic process requires amino acids as the raw material.
- All the naturally occurring proteins of living organisms fundamentally are the polymers of about 20 amino acids.
- These amino acids occur in the matrix forming an “amino acid pool” and are readily available for the process of protein synthesis.
- All amino acids present in the amino acid pool are called a-amino acids because one carbon, the a-carbon has four specific groups attached to it: an amino group, a carboxyl (acidic) group, a hydrogen, and one of the twenty different R groups (side chains), imparting the specific properties of that amino acid. (Technically, proline is called an imino acid because of its structure).
- Having these four groups attached to C imparts a property known as chirality on the amino acid: such as left and right-handed gloves, the mirror images cannot be superimposed. (Chirality means handedness or the property by which a form is distinguishable from its mirror image).
- Because of optical properties, the two forms of each amino acid are referred to as D and L, in which D comes from dextrorotatory (right turning) and L comes from levorotatory (left turning).
- All biologically active amino acids are of L form, and hence we need not refer to this designation.
- Proteins (polypeptides) are synthesized when peptide bonds form between any two amino acids- called residues when incorporated into a protein-can join and all chains will have an amino (N-terminal) end and a carboxyl (C-terminal) end.
Proteins (polypeptides) are synthesized by the formation of peptide bonds between two amino acids. The polypeptide chain synthesized in such a way will have an amino end (N-terminal) and a carboxyl (C-terminal) end.
Overview Of Mechanism Of Synthesis: The RNA-directed assembly of a protein is called translation and protein synthesis. The actual mechanism of protein synthesis can be divided into three stages:
- Initiation: The assembly of a ribosome on a mRNA molecule.
- Elongation: Repeated cycle of amino acid addition to a growing peptide chain.
- Termination: The release of the new protein chain. In a nutshell, the ribosome with its ribosomal RNA (rRNA) and proteins is the site of protein synthesis.
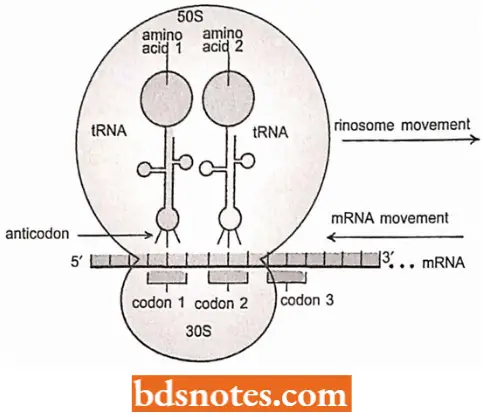
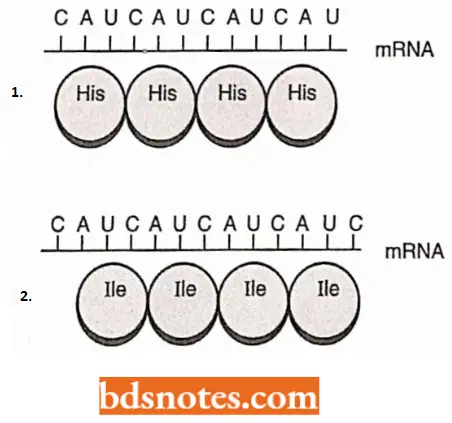
The information from the anticodon of the gene is in the form of messenger RNA (mRNA) in which each group of three nucleotides – a codon – specifies an amino acid.
- The amino acids are carried to the ribosome attached to transfer RNAs (tRNAs) and these transfer RNAs have anticodons, three nucleotides complementary to a codon, located at the end opposite the amino acid attachment site.
- A peptide bond will form between the two amino acids present at the ribosome, freeing one transfer RNA and lengthening the amino acid chain attached to the second transfer RNA.
- The messenger RNA will then move one codon concerning the ribosome, and a new transfer RNA will attach at codon 3.
.
- This cycle is then repeated, with the polypeptide lengthening by one amino acid each time.
- Significantly, the translation process starts precisely. Remember that the genetic code is translated into groups of three nucleotides (codons).
- If the reading of the messenger RNA begins one base too early or too late, the reading frame is shifted so that an entirely different set of codons is read.
- The protein produced, if any, will probably bear no structural or functional similarity to the protein the gene is coded for.
Mechanism Of Translation Or Protein Synthesis
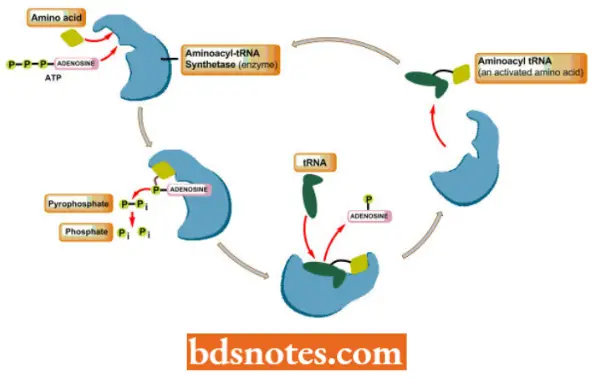
Aminoacylation of tRNA (Attachment of Amino Acid to Transfer RNA)
- The function of tRNA (transfer RNA) is to ensure that each amino acid incorporated into a protein corresponds to a particular codon in the messenger RNA. The transfer RNA serves this function through its peculiar structure: it has an anticodon at one end and an amino acid attachment site at the other end.
- The “correct” amino acid, the amino acid corresponding to the anticodon, is attached to the transfer RNA by enzymes known as aminoacyl tRNA synthetases (for example., arginyl tRNA synthetase, leucyl-tRNA synthetase).
- A transfer RNA with an amino acid attached is said to be “charged”. The transfer RNAs for each amino acid are designated by the convention tRNAUu (for leucine), tRNA, is (for histidine), and so on.
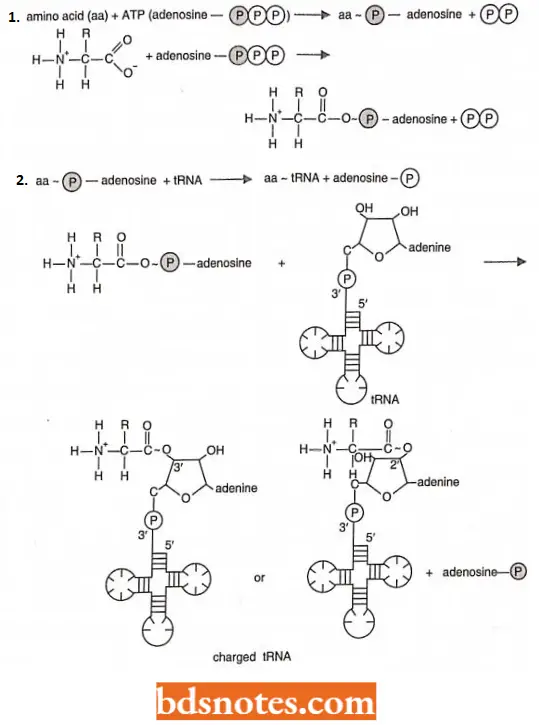
- An aminoacyl – tRNA synthetase joins a specific amino acid to its transfer RNA in a two-stage reaction that takes place on the surface of the enzyme In the first stage, the amino acid is activated with ATP.
- In the second stage of the reaction, the amino acid is attached with a high energy bond to the 2′ or 3′ carbon of the ribose sugar at the 3′ end of the transfer RNA:
The first step in aminoacyl – tRNA synthesis involves the activation of the amino acid using energy from ATP:

The aminoacyl ~ AMP intermediate is not normally released from the enzyme before undergoing the second step in aminoacyl tRNA synthesis, namely the reaction with appropriate tRNA:

- The resultant aminoacyl ~ tRNAs (amino acid ~ tRNAs) are immediate precursors of protein (= polypeptide) synthesis.
- In the, we denote high energy bonds, bonds that liberate a lot of free energy when hydrolyzed, as Thus, during the process of protein synthesis, the energy for the formation of the peptide bond will be present when it is needed, at the point of peptide bond formation.
Few Characteristics Of Aminoacyl-tRNA Synthetases: In bacteria, there are twenty aminoacyl-tRNA synthetases, one for each amino acid.
- A particular enzyme recognizes a particular amino acid and all the transfer RNAs that code for that amino acid.
- In eukaryotes, there are separate sets of twenty cytoplasmic and twenty mitochondrial aminoacyl-tRNA synthetases, all coded in the aminoacyl-tRNA synthetases, all coded in the nucleus.
- Aminoacyl-tRNA synthetases are a heterogeneous group of enzymes. In E.coli, these enzymes vary from monomeric proteins (one subunit) to tetrameric proteins, made up of two copies of each two subunits.
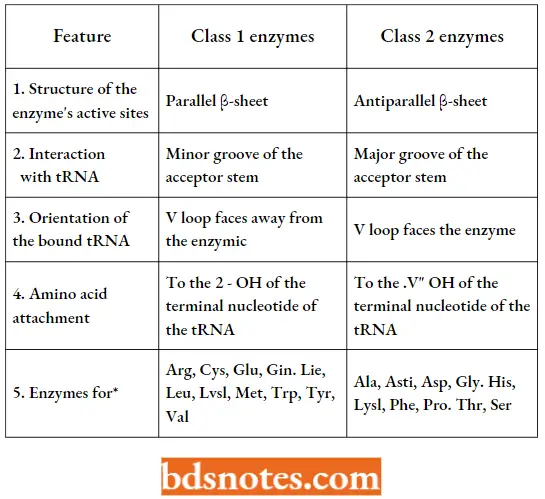
- The enzymes fall into two categories based on sequence similarity, structural features, and whether the amino acid is attached at the 2′-OH (in class I enzymes) or 3′-OH (in class 2enzymes) of the 3′-terminal adenosine of the transfer RNA.
- To add its appropriate amino acid to the appropriate transfer RNA, a synthetase recognizes many parts of transfer RNA.
- This can be demonstrated by experiments that introduce specific changes in transfer RNAs by site-directed mutagenesis.
- In seventeen of the twenty synthetase enzymes of E. coli, recognition involves part of the anticodon itself.
Features Of Aminoacyl-Trna Synthetases:
- Further, an aminoacyl-tRNA synthetase enzyme can initially make errors and attach the “wrong” amino acid to a tRNA. For example, isoleucine (lie) synthetase will attach valine about one in 225 times.
- This type of error occurs because a similar, but smaller, amino acid can sometimes occupy the active site of the enzyme (compare chemical formulae of isoleucine and valine.
- However, because of a proofreading step, only 1 in 270 to 1 in 800 of the errors are released intact from the enzyme.
- The amino acids on the rest of the incorrectly charged transfer RNAs are hydrolyzed before the transfer RNAs are released.
- Thus, only about one incorrectly charged transfer RNA occurs per 60,000 to 80,000 formed.
- In various bacteria, the number of amino acyl-tRNA synthetases in a particular organism is below twenty. For example, in some archaea, there is no cysteinyl-tRNA synthetase (enzyme).
- However, the prolyl tRNA synthetase activates the tRNAs for both cysteine and proline with their appropriate amino acids. Similarly, in some eubacteria, there is no glutaminyl-tRNA synthetase; the glutaminyl-tRNA is charged with glutamic acid, rather than glutamine.
- An amidotransferase (enzyme) then converts the glutamic acid to glutamine.
- This process is called transamidation. Some archaea also use the process of transamidation to synthesize asparagine-tRNAAsn from aspartic acid-tRNAAsn.
- Lastly, three of sixty-four codons are used to terminate translation.
- Thus, sixty-one transfer RNAS are needed because there are sixty-one different nonterminator codons.
- About fifty transfer RNAS are known in B. coli. The number can be explained by the Wobble phenomenon, which occurs in the third position of the codon.
Wobble helps in the reduction of several tRNAs: Aminoacylation represents the first level of specificity demonstrated by a tRNA.
- The second level is the specificity of the interaction between the anticodon of the tRNA and the codon of the mRNA being translated. This specificity ensures that protein synthesis follows the rules of the genetic code.
- In principle, codon-anticodon recognition is an easy process involving base-pairing between the anticodon of the tRNA and a codon in the mRNA.
- The specificity of aminoacylation ensures that the tRNA carries the amino acid denoted by the codon that it pairs with, and the ribosome controls the topology of the interaction in such a way that only a single triplet of nucleotides is available for pairing.
- Because base-paired polynucleotides are always antiparallel and because the mRNA is read in the 5’→3′ direction, the first nucleotide of the codon pairs with nucleotide 36 of the tRNA, the second with nucleotide 35 and the third with nucleotide 34.
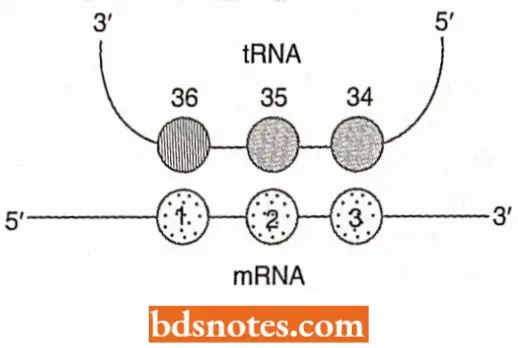
- All three nucleotides (i.e., 36, 35, and 34) form an anticodon. In practice, codon recognition is complicated by the possibility of Wobble. Because the anticodon is in a loop of tRNA, the triplet of nucleotides is slightly curved and so cannot make an entirely uniform alignment with the codon.
- As a result, a non-standard base pair can form between the third nucleotide of the codon and the first nucleotide (number 34) of the anticodon.
- This is called “wobble”. A variety of pairings is possible, especially if the nucleotide at position 34 is modified. In bacteria, the two main features of wobble are (Ikemura, 1981).
- G-U base pairs are permitted. This means an anticodon with the sequence 3′- XX G- 5′ can base-pair with both 5′ – XX C- 3′ and 5′- XX U- 3′. Similarly, the anticodon 3′- XX U- 5′ can base-pair with both 5′- XX A -3′ and 5′ – XXG – 3′.
- The consequence is that, rather than needing a different tRNA for each codon, the four members of the codon family [for example, 5′- GCN- 3′ (i.e., GCC, GCA, GCU, and GCG) all coding for alanine] can be decoded by just two tRNA.
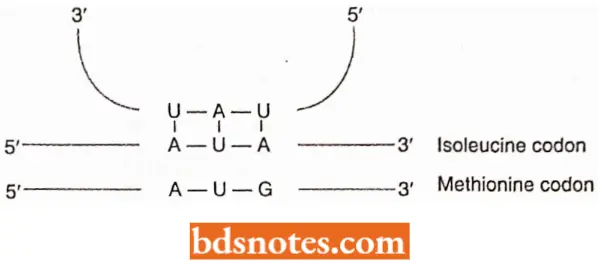
Inosine, abbreviated to I, is a modified purine that can base pair with A, C and U. Inosine can occur only in the tRNA because mRNA is not modified in this way. The triplet 3′- UAI- 5′ is sometimes used as the anticodon in a tRNA Ile because it pairs with 5′- AUA – 3′, 5′- AUC- 3′, and 5′- AUU- 3′, which form the three codon family for this amino acid (i.e. Isoleucine) in the standard genetic code.
- Thus, wobble tends to reduce the number of tRNAs in a cell by enabling one tRNA to read two or possibly three codons. Hence, bacteria can decode their mRNAs with as few as 30 IRNAs. Eukaryotes also make use of wobble but in a restricted way. For example, the human genome has 48 tRNAs.
- Of these, 16 tRNAs are predicted to use wobble to decode two codons each, with the remaining 32 tRNAs being specific for just a single triplet
- In eukaryotes, the bacterial G- U wobble is used with eight tRNAs but in every case, the wobble involves an anticodon with the sequence 3′ – XXG – 5′.
- The alternative version of G- U wobble, where the anticodon sequence is 3′- XXU- 5′, appears not to be used in eukaryotes, possibly because this could result in a tRNA, lc with the anticodon 3′- UAU – 5′ reading the methionine codon 5′ -AUG- 3′. Eukaryotes may therefore have a means of preventing this type of wobble from occurring.
- Eight other human tRNAs have anticodons containing inosine (3′- XXI- 5′) but, these decode only 5′ – XXC- 3′ and 5′ – XXU – 3′ codons. The base pairing between and A is weak, which means that 5′- XXA- 3′ codons are only inefficiently recognized by a 3′- XXI – 5′ anticodon.
- To avoid this inefficiency, in every example of wobble involving inosine in the human tRNA set, the 5′- XXA – 3′ codon is recognized by a separate tRNA.
- Note, however, that recognition by a separate tRNA does not preclude the 5′- XXA- 3′ codon from also being decoded by the tRNA 3′ — XXI- 5′, albeit inefficiently.
- This does not compromise the specificity of the genetic code, because wobble involving inosine is limited to those codon families in which all four triplets specify the same amino acid.
Superwobble: Human mitochondria use only 22 tRNAs, with some of these tRNAs the nucleotide in the wobble position of the anticodon is virtually redundant because it can base with any nucleotide, enabling all four codons of a family to be recognized by the same tRNA. This phenomenon has been called superwobble.
Experiment To Show That The Transfer RNA, Not The Amino Acid, Is Recognized During Protein Synthesis:
- Chapeville and colleagues, in 1962, isolated transfer RNA with cysteine attached.
- They chemically converted the cysteine to alanine by using Raney nickel, a catalytic form of nickel that removes the SH group of cysteine.
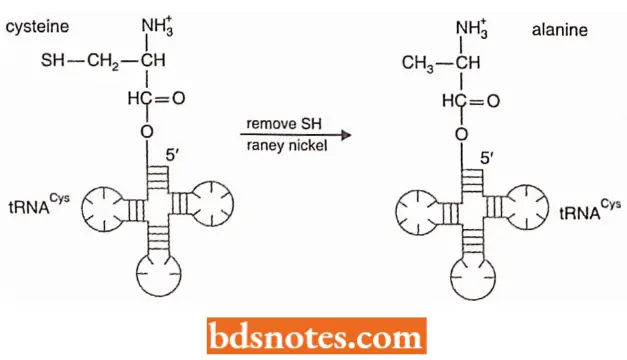
- When such modified tRNAs were used in protein synthesis, alanine was incorporated where cysteine should have been.
- This result demonstrated that the transfer RNA, not the amino acid, was recognized during protein synthesis.
- Thus, the synthetase puts a specific amino acid on a specific tRNA; then, during protein synthesis, the anticodon on the tRNA- not the amino acid itself- determines which amino acid is incorporated.
Initiation Of Translation
Initiation In prokaryotes: Interaction between initiation codon and formylmethionine- tRNA.
- An important feature of the initiation of polypeptide ( = protein) synthesis is the use of a specific initiating tRNA molecule.
- In Escherichia coli and other prokaryotes, the synthesis of every protein begins with the modified amino acid N-formyl methionine.
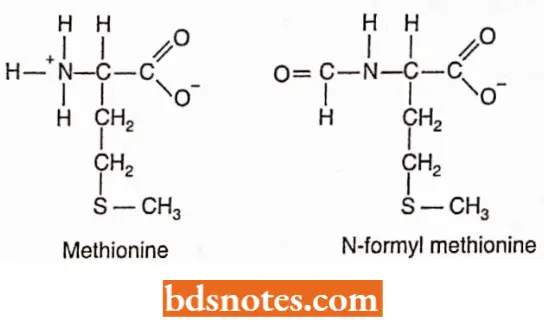
- However, none of the completed protein in E.coli contains N-formyl methionine. Many of these proteins do not even have methionine as their first amino acid. Before a protein becomes functional, the initial amino acid is modified or removed (called processing).
- In eukaryotes, the initial amino acid is also methionine but it does not have a N-formyl group.
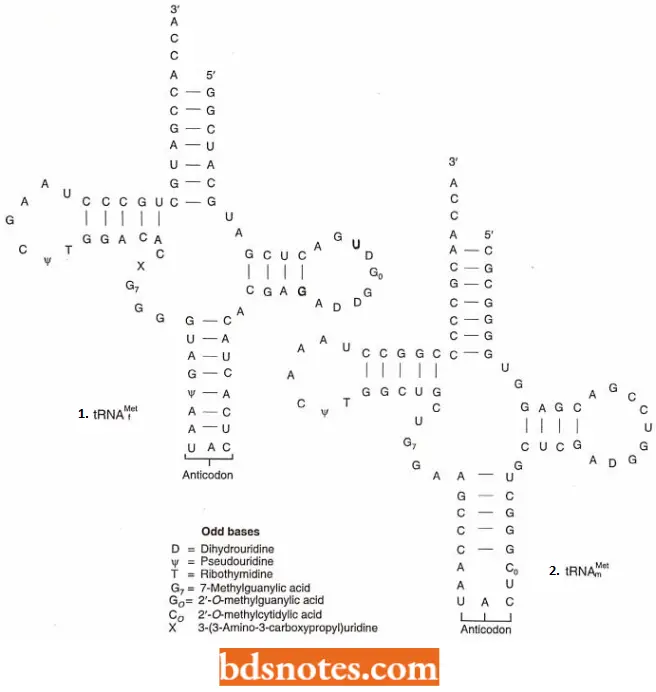
- The codon for methionine is 5′- AUG- 3′ and is called the initiation codon. Methionine has two tRNAs with the same anticodon (3′- UAC- 5′) but different structures. One of these tRNAs (tRNAfMet) is part of the initiation complex.
- Before the initiation of translation, this tRNA will have its methionine chemically modified to N-formyl methionine. The other transfer RNA will not have its methionine modified (tRNAmMet).
- The translation process will use it to insert methionine into proteins, where it is required, in all but the first position.
- The cell thus has a device to make use of methionine in the normal way as well as to use a modified form of it (i.e., formyl methionine) to initiate protein synthesis.
Because of the structure of the prokaryotic initiation transfer RNA, it can recognize AUG, GUG, and rarely UUG as initiation codons. In eukaryotes, CUG as well as AUG can serve as an initiation codon.
- Since the initiation methionine is not formylated in eukaryotes, the eukaryotic transfer RNA is designated tRNAjmel; there is a separate internal methionine transfer RNA, termed tRNAmMet, in eukaryotes as well as prokaryotes.
- Since in protein synthesis, the peptide chain always grows in a sequence from the free terminal amino (-NH2) group towards the carboxyl (—COOH) end, the function of formyl methionine tRNA is to ensure that proteins are synthesized in that direction.
- In the formylmethionine- tRNA, the amino (-NH2) group is blocked by the formyl group leaving only the -COOH group available to react with the -NH2 group of the second amino acid (AA2). In this way, the synthesis of the protein chain follows in the correct sequence.
- Formation of initiation complex The 30S and 50S ribosomal subunits of bacteria usually dissociate from each other when not involved in translation.
- To begin translation, an initiation complex forms, consisting of the following components in prokaryotes (i.e., E.coli): the 30S subunit of the ribosome, a messenger RNA, the charged N-formyl methionine tRNA (fmet – tRNAfMet), and three initiation factors (IF1, IF2, IF3).
- Initiation factors (as well as elongation and termination factors) are proteins loosely associated with the ribosome.
- They were discovered when ribosomes were isolated and then washed, losing the ability to perform translation (i.e., polypeptide synthesis).
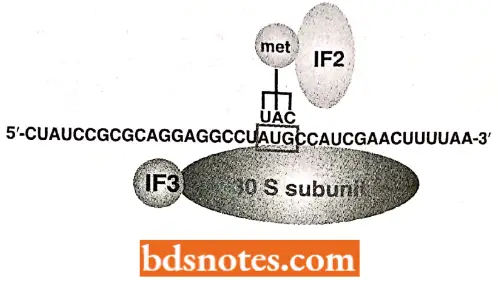
Various components of the initiation complex interact in a series of steps. It is known that IF3 binds to the 30S ribosomal subunit, allowing the 30S subunit to bind to messenger RNA. Meanwhile, complex forms with IF2, the charged N-formylmethionine tRNA (frnet- tRNAfMet), and GTP (guanosine triphosphate;
Functions Of The Bacterial ( =Prokaryotic) Translation Factors:
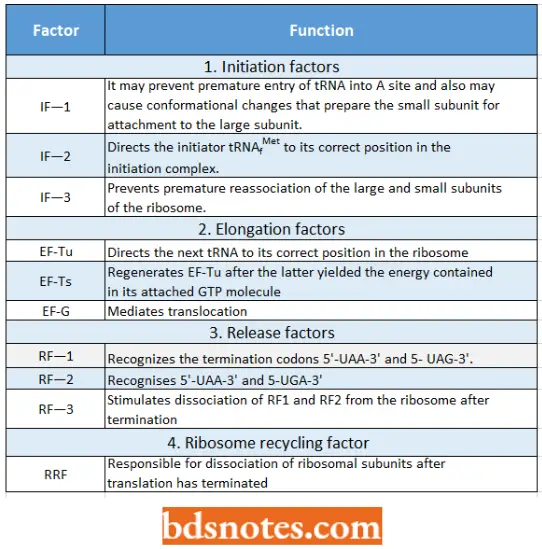
- It is IF2 that brings the initiator transfer RNA to the ribosome. IF2 binds only to the charged initiator transfer RNA, and without IF2, the initiator tRNA cannot bind to the ribosome. The final step in initiation-complex formation is bringing together the first two components.
- Here, GTP has unusual function. The hydrolysis of GTP to GDP + Pi (inorganic phosphate, the P04~3 ) produces conformational changes; these changes allow the initiation complex to join the 50S ribosomal subunit to form the complete ribosome and then allow the initiation factors and GDP to be released.
- Generally, the hydrolysis of a nucleoside triphosphate (i.e., ATP, GTP) in a cell occurs to release the energy in the phosphate bonds for use in a metabolic process.
- However, in the process of translation, the hydrolysis of GTP changes the shape so that it and the initiation factors can be released from the ribosome after the 70S particle has been formed.
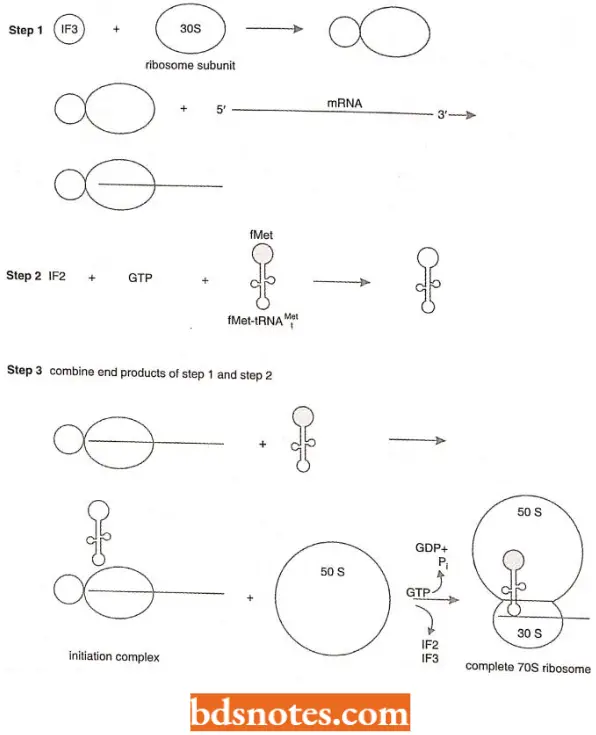
- Thus, hydrolysis of GTP in translation is for conformational change rather than covalent bond formation. IF1 helps the other two initiation factors bind to the 30S initiation complex.
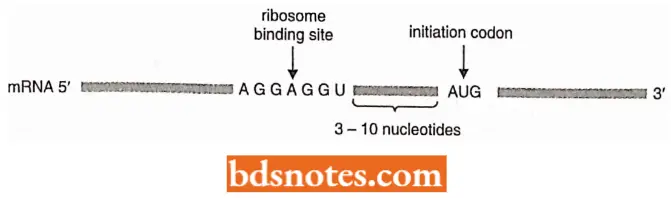
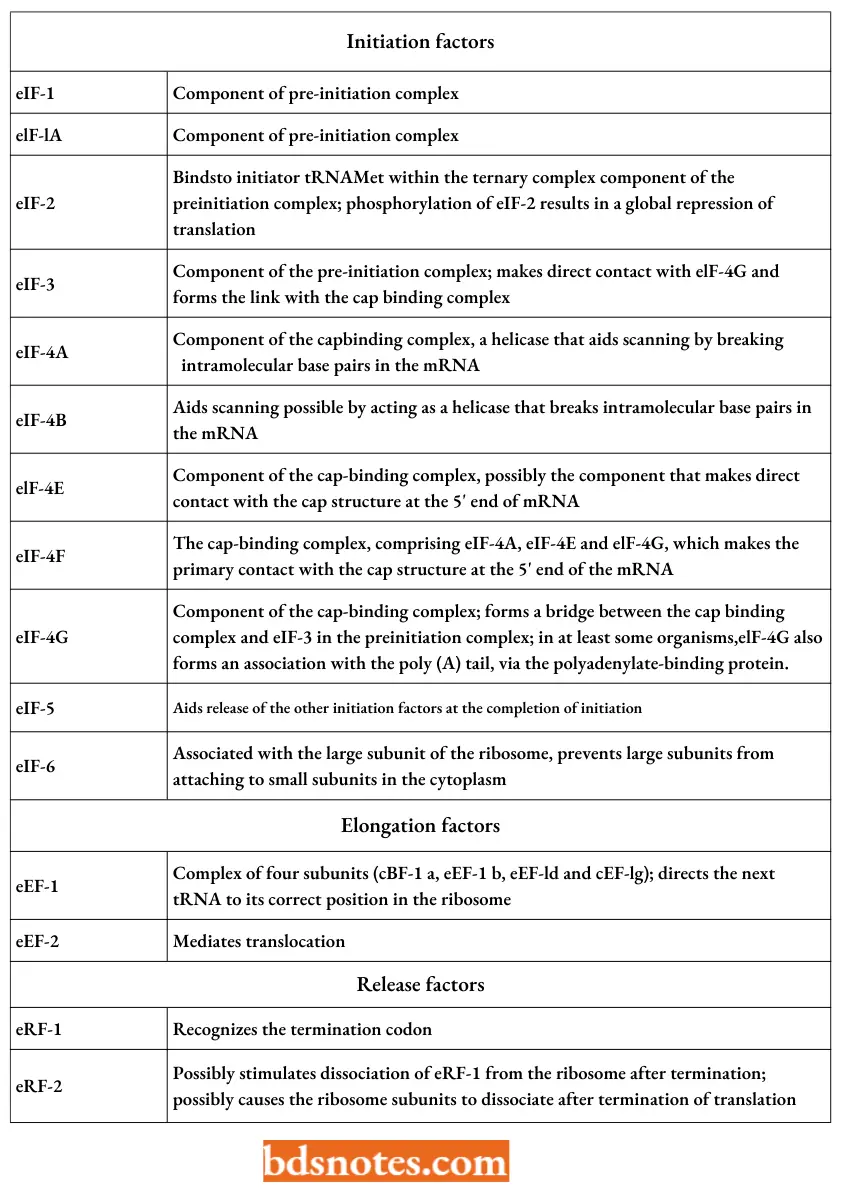
- How does docs 70S ribosome recognize mRNA? The ribosome recognizes the prokaryotic mRNA through complementarity of a region at the 3′ end of the 16S ribosomal RNA and a region slightly upstream from the initiation sequence (AUG) on the mRNA.
- This consensus sequence is 5′ -AGGAGGU- 3′ and is called the Shine-Dalgarno sequence. It is located between 3 to 10 nucleotides upstream of the initiation codon.
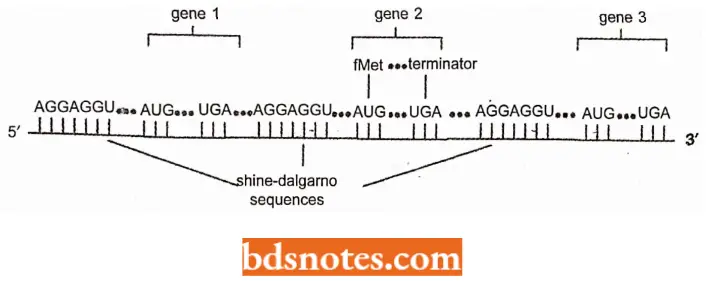
Moreover, in prokaryotes, most mRNAs contain the information for several genes. These RNAs are called polycistronic mRNAs. Each gene on the messenger RNA is translated independently: each gene has a Shine- Dalgamo sequence for ribosome recognition and an initiation codon (AUG) for fMet.
Initiation In Eukaryotes: The process of initiation complex formation in eukaryotes is generally similar to prokaryotes but more complex.
- The eukaryotic initiation factor abbreviations are preceded by an “e” to denote that they are eukaryotic (elFl, eIF2, etc.).
- At least eleven initiation factors are involved, including a specific cap-binding protein, eIF4E. The Shine-Dalgarno sequence is altogether absent in eukaryotes.
- The actual mechanism for recognizing the 5′ end ofeukaryotic messenger RNA appears to be based on the recognition of the 5′ cap of the messenger RNA by the cap-binding protein with recruitment of other initiation factors and the small subunit of the ribosome.
- This is followed by the small subunit’s movement down the messenger RNA. The ribosome scans the RNA until it recognizes the initiation codon. This model is known as the scanning messenger hypothesis.
- In many eukaryotes, a process called shunting occurs, in which the first AUG does not serve as the initiation codon; rather, scanning begins, but it bypasses a region of the messenger RNA upstream of the initiation codon, called the leader or 5′ untranslated region (5’UTR), in favor of an AUG further down the messenger RNA.
- The cause of shunting seems to be a secondary structure in the messenger RNA, upstream from the AUG codon that serves as the initiation codon.:
- In some eukaryotes, very small genes called open reading frames (ORFs) are present in this region of the messenger RNA and play some role in shunting.
- In the genes of some plant and animal viruses, ORFs are translated, and then the main gene is translated by the same ribosome in a process called reinitiation.
- In some eukaryotes, ribosomes can initiate protein synthesis within the mRNA if that mRNA contains a sequence called an IRES (i.e., internal I ribosome entry site).
- These sequences were discovered in the RNA genome of the human poliovirus and rhinovirus (for B cold) and in several cellular messenger RNAs. They are at least four hundred nucleotides long.
Eukaryotic Translation Factors:
Initiation Of Eukaryotic Translation With Scanning: The first step involves the assembly of the pre-initiation complex.
- This structure comprises the 40S ribosomal subunit, a ‘ternary complex’ made up of the initiation factor eIF-2 bound to the initiator tRNAMet and a molecule of GTP, and three additional initiation factors, eIF-1, elF-lA, and eIF-3.
- As in bacteria, the initiator tRNA is distinct from the normal tRNAMet that recognizes.
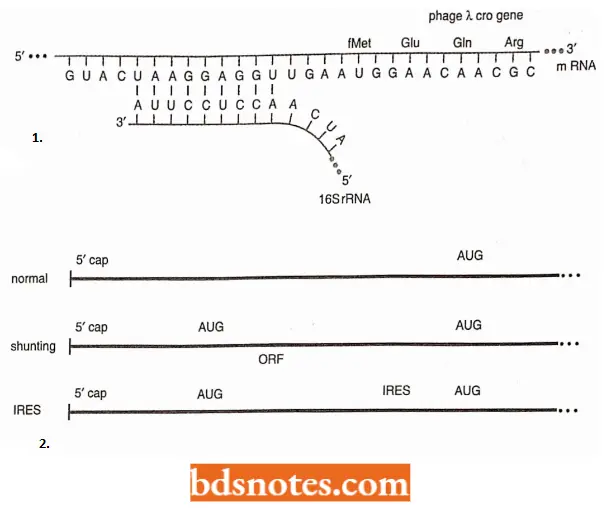
- After assembly, the pre-initiation complex associates with the 5′ end of the mRNA. This step requires the cap-binding complex (sometimes called eIF-4F) which comprises the initiation factors elF-4A, eIF-4E, and eIF-4G. The contact with the cap might be made by eIF-4E alone or involve a more general interaction with the cap-binding complex (Pestova and Hellen, 1999).
- The factor eIF-4G serves as a bridge between eJF-4E, bound to the cap, and eIF-3, attached to the initiation complex gets attached to the 5′ region of the mRNA. Attachment of the pre-initiation complex to the mRNA is also influenced by the poly tail, at the distant 3′ end of the mRNA.
- This interaction is thought to be mediated by the polyadenylate-binding protein (PADP) attached to the poly tail.
- In yeast and plants, PADP is found to form an association with eIF-4G and for such an association mRNA has to bend back on itself.
With its attachment to the 5′ end of the mRNA, the initiation complex has to scan along the mRNA molecule and find the initiation codon.
- The leader regions of eukaryotic mRNAs can be several tens or even hundreds of nucleotides in length and often contain regions that form hairpins and other base-paired structures.
- These are probably removed by a combination of- 4A and eIF-4B. The eIF-4A, and possibly also eIF-4B, has helicase activity and can break intra¬ molecular base pairs in the mRNA, freeing the passage for the initiation complex.
- The initiation codon (AUG) is recognizable because it is contained in a short consensus sequence; 5′ ACCAUGG-3′, which is called Kozak consensus.
- As soon as the initiation complex is positioned over the initiation codon, the large subunit (60S) of the ribosome attaches.
- As in bacteria, this requires hydrolysis of GTP and leads to the release of the initiation factors.
- Two final initiation factors are involved at this stage: eIF-5 which aids release of the other factors and eIF-6, which is associated with the unbound large subunit and prevents it from attaching to a small subunit in the cytoplasm.
Initiation Of Eukaryotic Translation Without Scanning: Transcripts (mRNA) of picornaviruses (i.e., RNA viruses such as poliovirus and rhinovirus) are not capped but instead have an internal ribosome entry site (IRES).
- The presence of IRES on their transcripts means that picoma viruses can block protein synthesis in the host cell by inactivating the cap-binding complex, without affecting translation of their transcripts.
- Quite interestingly, no virus proteins are required for the recognition of an IRES by a host ribosome. In other words, the normal eukaryotic cell possesses proteins and or other factors that enable it to initiate translation by the IRES method (Holick et al., 2000). Some nuclear genes are reported to have IRES.
- For example, IRES exist on mRNAs for the mammalian immunoglobulin heavy chain binding protein and the Drosophila antennapedia protein. IRES are also found on several mRNAs whose protein products are translated when the cell is put under stress, for example by exposure to heat, irradiation, on low oxygen conditions.
- Under these circumstances, cap-dependent translation is globally suppressed.
- The presence of IRESs on the ‘survival’ mRNAs therefore enables these to undergo preferential translation at the time when their products are needed.
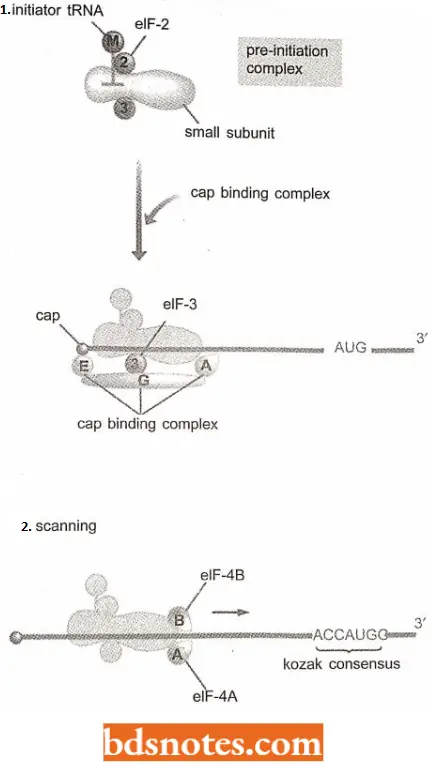
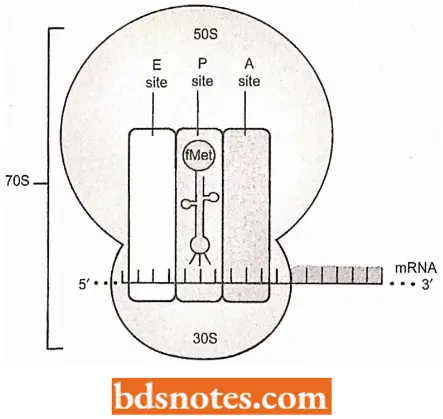
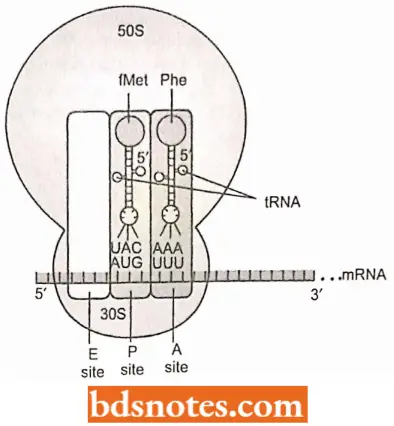
Three Sites of Ribosomes: When the initiator transfer RNA joins the 30S ribosomal subunit of prokaryotes with its mRNA attached, it fits into one of three sites in the ribosome.
- These sites or cavities in the ribosome, are referred to as the aminoacyl site (A site), the peptidyl site (P site), and the exit site.
- Here we concentrate on A and P sites, each of which contains a transfer RNA just before forming a peptide bond: the P site contains the transfer RNA with the growing peptide chain (peptidyl – tRNA); the A site contains a new transfer RNA with its single amino acid (aminoacyl-tRNA).
- The E site helps eject the depleted transfer RNAs after a peptide bond forms. When a complete 70S ribosome has formed, the initiation fMet- tRNAfMet is placed directly into the P site, the only charged tRNA that can be placed there.
- The association of tRNA and ribosome is aided by a G-C base pairing between the 3′- CCA terminus of all transfer RNAs and a guanine in the 23S ribosomal RNA.
Elongation: The main differences between translation in bacteria and eukaryotes occur during the initiation phase; the events after the large subunit of the ribosome becomes associated with the initiation complex are similar in both types of organisms.
Positioning a second transfer RNA: The second charged tRNA binds (due to a codon-directed binding) to the first ribosome at the latter s A site with the help of the proteins, called elongation factors ((for example. EF-Tu). EF-Tu carries a molecule of GTP.
- Coned hydrogen bonding with the mRNA template dictates the selection of a new tRNA, and the activity of the HF-Tu ensures the proper positions of the tRNA in the A
site. - Such a placement activity needs energy that is provided by the hydrolysis of the molecule of
6 IT to GUI’ and phosphate. - After performing its function, the KF-Tu protein dissociates from the ribosome, and in the cytoplasm is subsequently regenerated to its active form by another elongation factor, the EF-Ts.
- At this point, both sites of the ribosome are occupied by RNA’s each of which carries an amino acid, and each of which is hydrogen bonded to the template mRNA.
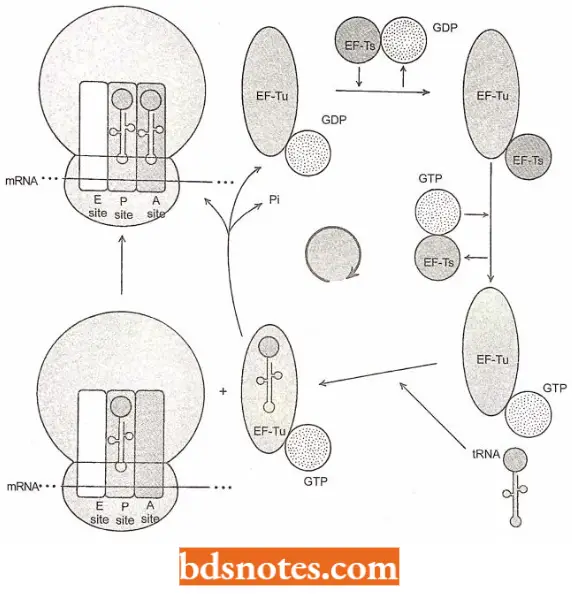
The structures revealed by X-ray crystallography show that A, P, and E sites are located in the cavity between the large and small subunits of the ribosome, the codon-anticodon interaction being associated with the small subunit and the aminoacyl end of the mRNA with the large subunit.
- Further, it takes several milliseconds for the GTP to be hydrolyzed, and another few milliseconds for the EF-Tu/GDP to leave the ribosome.
- During those two intervals of time, the codon-anticodon fit of the transfer RNA is scrutinized. If the correct tRNA is in place, a peptide bond forms.
- If not, the charged tRNA is released and a new cycle of EF-Tu/GTP-mediated testing of tRNAs begins. The error rate is only about one mistake in ten thousand amino acids incorporated into protein.
- The speed of amino acid incorporation is about fifteen amino acids per second in prokaryotes and about two to five per second in eukaryotes.
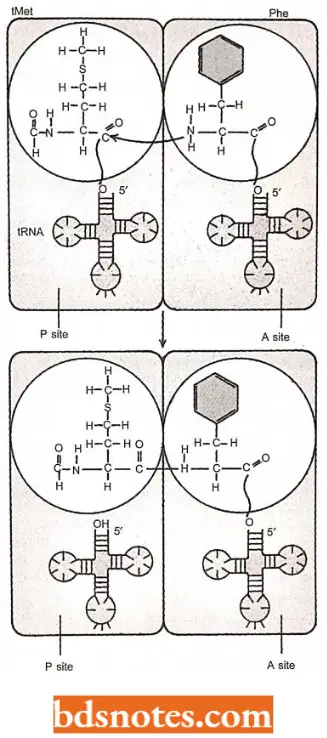
Peptide bond formation: The next step is the formation of a peptide bond between the two amino acids. To accomplish this job, the first amino acid (N-formylmethionine) is removed from its attachment to its tRNA and transferred to the free-NH2 terminus of the second amino acid.
- The first amino acid is, thus, placed “on top of’ the second. The ensuing peptide bond, thus, joins the carboxyl group of the first amino acid with the amino group of the second amino acid.
- The resulting compound is a dipeptide whose carboxyl end is still bonded to the second tRNA, but whose amino end is free. The reaction is catalyzed by an enzyme associated with the 50S subunit and called peptidyl transferase.
- This enzymatic center, an integral part of the 50S subunit, was originally believed to be composed of parts of several of the 50S proteins. Now, however, it is believed to have ribozyme activity (enzymatic activity) of 23S rRNA of 50S ribosomal subunit.
- The enzymatic activity involves a bond transfer from the carboxyl end of N-formyl .methionine to the amino end of the second amino acid. Every subsequent peptide bond is identical, regardless of the amino acids involved.
- The energy used is contained in the high-energy ester bond between the transfer RNA in the P site and its amino acid. Immediately after the formation of the peptide bond, the transfer RNA with the dipeptide is in the A site, and a depleted tRNA is in the P site.
Translocation: The next stage in elongation is the translocation of the ribosome between the tRNA and the mRNA.
- Elongation factor EF-G, earlier called translocase, catalyzes the translocation process.
- The ribosome must be converted from the pretranslocational state to the post-translocational state by the action of EF-G, which physically moves the mRNA and its associated transfer RNA.
Thus, during translocation following three things happen at once:
- The ribosome moves along the three nucleotides so that the next codon enters the A site.
- The dipeptide-tRNA in the A site moves to the P site.
- The deacylated tRNA in the P site moves to a third position, the E or exit site in bacteria or in eukaryotes, is simply ejected from the ribosome.
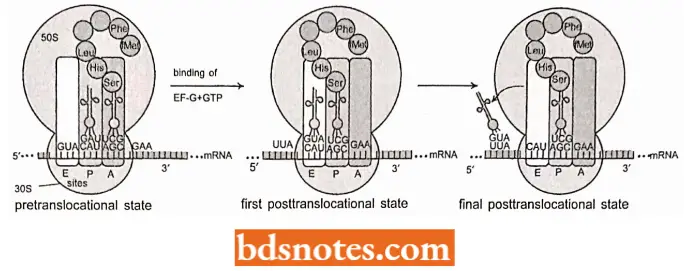
- Translocation requires hydrolysis of a molecule of GTP. Electron microscopy of ribosomes at different intermediate stages in translocation suggests that the two subunits rotate slightly in opposite directions, opening up the space between them and enabling the ribosome to slide along the mRNA (Frank and Agarwal, 2000).
- The sequential formation of a polypeptide continues in the manner described above. A tRNA in the ‘P’ site shifts its burden of growing polypeptide to the next succeeding tRNA, followed by translocation, exit of the discharged tRNA, and entrance of a new charged tRNA (having correct anticodon) to base pair with a new codon at ‘A’ site.
- Thus, the growing polypeptide is adopted by each tRNA, with each successive amino acid being added in effect, to the bottom of the stack.
- As the process continues, the mRNA is progressively translated, codon by codon, from the 5′ end to the 3′ end.
- In eukaryotes, three elongation factors perform the same tasks that EF-Tu, EF-Ts, and EF-G perform in prokaryotes.
- The factor eEFla replaces EF-Tu, factor eEFlBy replaces EF-Ts and eEF2 replaces EF-G. According to Brown (2002), elongation factor eEF-1 is a complex of four subunits: eEF-1 a eEF-lb, eEF-ld, and eEF-1 g.
- The first of these exists in at least two forms, eEF-lal, and eEF-1a2, which are similar proteins that probably have equivalent functions in different tissues (Hafezparast and Fisher, 1998).
Termination: Termination of translation (or protein synthesis) in both prokaryotes and eukaryotes occurs when one of three nonsense codons appears in the A site of the ribosome. These codons are UAG (amber), UAA (ochre) and UGA (opal).
- In prokaryotes, three proteins called release factors (RF) are involved in termination, and a GTP is hydrolyzed) to GDP + Pi. When a nonsense codon enters the A site on the ribosome, a release factor recognizes it.
- RF1 and RF2 are class 1 release factors: they recognize termination/stop codon and then promote hydrolysis of the bond between the terminal amino acid and its tRNA in the P site. Class 2 release factors (for example RF3) do not recognize stop codons, but they stimulate class 1 release factors to act.
- RF1 recognizes the termination or stop codon UAA and UAG and RF2 recognizes UAA and UGA.
- Both do so because they have tripeptides that mimic anticodons to recognize the stop codon: proline-alanine-threonine in RF1 and serine-proline-phenylalanine in RF2.
- In this molecular mimicry, a protein mimics the shape of a nucleic acid to function properly. The next base in mRNA past the stop codon is usually an adenine, required for efficient termination. After the release factors act, with the hydrolysis of a GTP, the ribosome has completed its task of translating mRNA into a polypeptide.
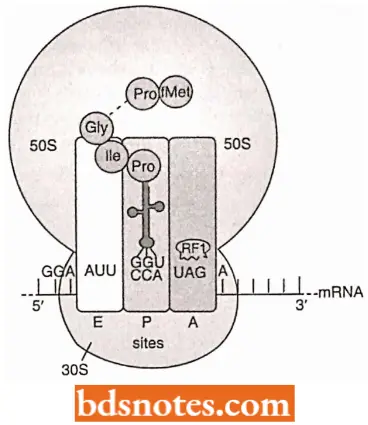
- The final release of all factors and dissociation of the two subunits of the ribosome takes place with the help of IF3, which rebinds to the 30S subunit, and a ribosome recycling factor (RRF). RRF has a tRNA-like structure like the eRF-1 (Selmer et al., 1999).
- RRF probably enters the P or A site and “unlocks” the ribosome. Dissociation requires energy, which is released from GTP by EF-G, and also requires the initiation factor IF-3 to prevent the subunits from attaching again.
- The disassociated ribosome subunits enter the cytoplasmic pool, where they remain until used again in another round of translation.
- Eukaryotes have just two release factors: eRF-1, which recognizes the termination codon, and eRF-3, which might play the same role as RF-3 although this has not been proven.
- The structure of eRF-1 has been solved by X-ray crystallography, showing that the shape of this protein is very similar to that of a tRNA (Kissclev and Buckingham, 2000). Further, in contrast to the often polycistronic mRNA of bacteria, mRNA of eukaryotes are monocistronic, containing the coding sequence only for one polypeptide.
- The initiation codon for this sequence is located near the 5′ end of the message. Thus, the association of mRNA takes place on the 5′ end and not at the initiation codon AUG as in prokaryotes. comparison of Prokaryotic and Eukaryotic Translation.
Comparison Between Prokaryotic And Eukaryotic Translation:
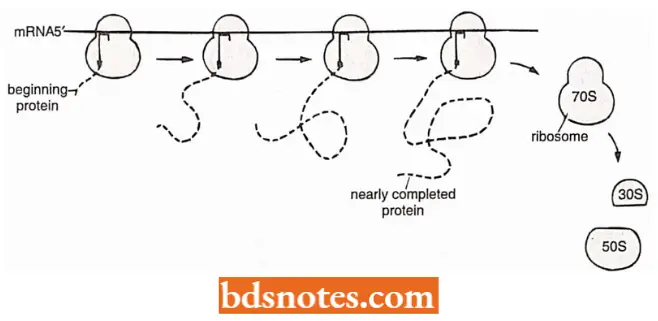
Polysomes and Coupled Transcription-Translation: The translation unit is not simply a ribosome traversing a ntRNA molecule, but is a more complex structure.
- After about 25 amino acids have been joined in a polypeptide chain, the AUG initiation site of the encoding mRNA molecule is completely free of the ribosome.
- A second initiation complex then forms. The overall configuration is of two 70S ribosomes moving along the mRNA at the same speed. When the second ribosome has moved along a distance similar to that traversed by the first, a third ribosome can attach.
- This process, i.e., movement and reinitiation, continues until the mRNA is covered with ribosomes at a density of about one 70S ribosome per 80 nucleotides.
- This large translation unit is called a polyribosome or simply a polysome. This is the usual form of translation unit in all cells.
- The use of polysomes is advantageous to a cell since the overall rate of protein synthesis is increased compared to the rate that would occur if there were no polysomes.
- Further, a mRNA molecule being synthesized has a free 5′ terminus and translation also occurs in the 5′ → 3′ direction, so each cistron contained in the mRNA immediately starts its translation.
- As a result, the ribosome binding site is transcribed first, followed in order by the AUG codon, the region encoding the amino acid sequence, and finally the stop codon.
- Thus, in bacteria with no nuclear membrane separating the DNA and the ribosomes, there is no obvious reason why the 70S initiation complex should not form before the mRNA is released from the DNA.
- With prokaryotes (E.coli) this does indeed occur; this process is called coupled transcription-translation.
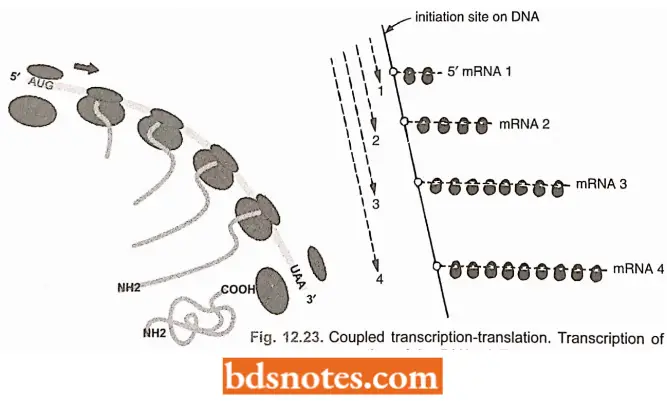
- This coupled activity does not occur in eukaryotes, because the mRNA is synthesized and processed in the nucleus and later on transported through the nuclear membrane to the cytoplasm where the ribosomes are located.
- Coupled transcription-translation, too, speeds up protein synthesis in the sense that translation does not have to await the release of mRNA from the DNA.
Unsual Events During Translation Elongation
The simple codon-by-codon translation of an mRNA is viewed as the standard way in which proteins are synthesized. But following three unusual translation elongation events have been reported:
- Frameshifting;
- Translation slippage; and
- Translation bypassing
1. Frameshifting: This event occurs when a ribosome pauses in the middle of an mRNA, moves back one nucleotide or, less frequently, forward one nucleotide, then continues translation (Farabaugh, 1996).
The result is that the codonsτ that are read after the pause are not contiguous with the preceding set of codons, i.e., they lie in a different reading frame. Such frameshifts may be spontaneous or programmed.
Spontaneous Frameshifts: They occur randomly and are deleterious because the polypeptide synthesized after the frameshift has the incorrect amino acid sequence.
Programmed Frameshifts: They occur only in some mRNAs. A few mRNAs utilize programmed frameshifting to induce the ribosome to change frame at a specific point within the transcript.
- Programmed frameshifting occurs in all types of organisms, from bacteria to humans, as well as during the expression of several viral genomes.
- An example occurs during the synthesis of DNA polymerase 3 (enzyme) in E. coli, the main enzyme involved in the replication of DNA. Two of the subunits of DNA polymerase 3, γ (gamma) and τ (tau), are coded by a single gene, dnaX.
- Subunit τ is the full-length translation product of the dnaX mRNA, and subunit y is a shortened version.
- Synthesis of subunit involves a frameshift in the middle of dnaX mRNA, the ribosome encountering a termination codon immediately after frameshift, producing the truncated y version of the translation product.

It is thought that the frameshift is induced by three features of the dnaX mRNA:
- A hairpin loop, located immediately after the frameshift position, stalls the ribosome.
- A sequence is similar to a ribosome binding site immediately upstream of the frameshift position, which is thought to base-pair with the 16S rRNA, again causing the ribosome to stall.
- The codon 5′ -AAG-3′ at the frameshift position. The presence of a modified nucleotide at the wobble position of the tRNAlys that decodes 5′ -AAG-3′ means that the codon-anticodon interactions is relatively weak at this position, enabling the frameshift to occur.
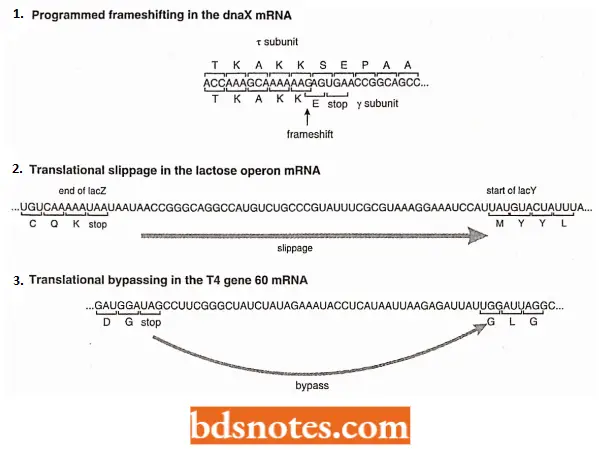
2. Translation Slippage: The phenomenon of translation slippage enables a single ribosome to translate an mRNA that contains copies of two or more genes.
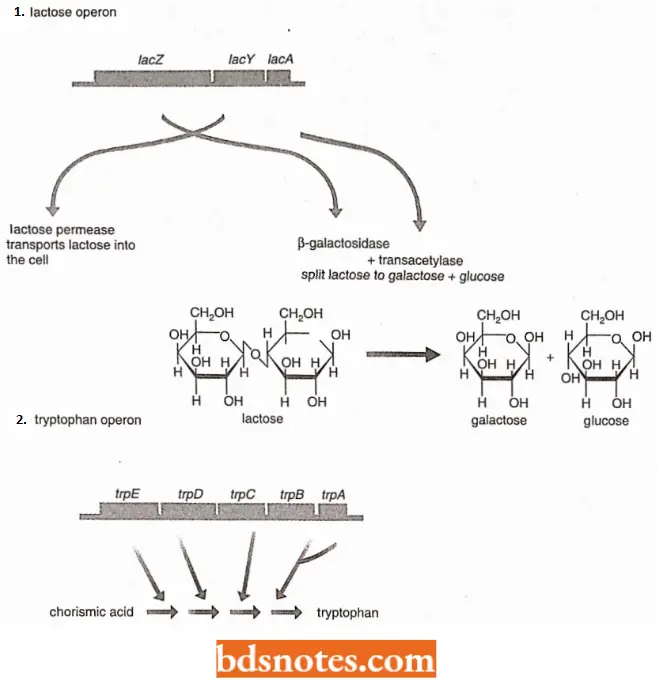
- Due to this phenomenon, a single ribosome can synthesize each of the five proteins coded by the mRNA transcribed from the tryptophan operon of E. coli.
- When the ribosome reaches the end of one series of codons ((for example., UAA), it releases the protein it has just made, and slips to the next initiation codon ((for example., AUG), and begins synthesizing the next protein.
3. Translation Bypassing: It is an extreme form of translation slippage. In translation bypassing, a larger part of the transcript, possibly a few tens of base pairs, is skipped, and elongation of the original protein continues after the bypassing event.
- The bypass starts and ends either at two identical codons or at two codons that can be translated by the same tRNA by wobble.
- This suggests that the jump is controlled by the tRNA attached to the growing polypeptide, which scans the mRNA as the ribosome tracks along and halts the bypass when a new codon to which it can base-pair is reached.
- Translation bypassing of 44 nucleotides occurs in E.coli during translation of the mRNA for gene 60 of T4 bacteriophage, which codes for the DNA topoisomerase subunit. Similar events have also been identified in a variety of other bacteria.
- Bypassing could result in two different proteins being synthesized from one mRNA- one protein from normal translation and one from bypassing, but whether this is its general function is not yet known.
Antibiotics And Protein Synthesis
Many antibacterial agents (called antibiotics) have been isolated from fungi. Most of these are inhibitors of protein synthesis.
- For example, streptomycin and neomycin bind to a particular protein in the 30S particle and thereby prevent binding of tRNAfMet to the ‘P’ site; the tetracyclines inhibit binding of charged tRNA; lincomycin and chloramphenicol inhibit the peptidyl transferase: and puromycin causes premature chain termination; erythromycin binds to a free 50S particle and prevents the formation of the 70S ribosome.
- A particular antibiotic has clinical value only when it acts on bacteria and not on animal cells; the clinically useful antibiotics usually either fail to pass through the cell membrane of animal cells or do not bind to eukaryotic ribosomes, because of some unknown feature of their structure.
- Some disease-causing bacteria exert their pathogenic effect because they excrete inhibitors of mammalian protein synthesis.
- The agent causing diphtheria is an example; it binds to a factor necessary for the movement of mammalian ribosomes along the mRNA.
Certain Other Poisons Of Cells
- The antibacterial drug nalidixic acid is a DNA replication inhibitor; it binds with the gyrase A subunit.
- The antibacterial drug novobiocin is also a DNA-; replication inhibitor; it binds to the gyrase B subunit and inhibits ATP cleavage.
- The centrosome duplication is arrested in the mammalian cells by the antibiotic aphidicolin. DNA polymerases L, S, and E are sensitive to inhibition by aphidicolin.
- Microtubular poisons are benomyl and nocodazole.
Translation Protein Synthesis Questions And Answers
Question 1. How many different mRNAs could specify the amino acid sequence met-phe-ser-pro?
Answer: 1×2×6×2 = 48
Question 2. Using the information in Table 10.2 convert the following mRNA segments into their polypeptide equivalents:
- …. 5’GAAAUGGCAGUUUAC3’….;
- …..3UUUCGAGAUGUCAA 5’……; and
- …..5’AAA ACC UAG AAC CCA3′
Answer:
- -Glu-met-ala-val-tyr-.
- -Phe-ala-arg-cys-asn-(since genetic code is always read in 5′ -4 3′ direction).
- -Lys-thr- (nonsense), chain terminates prematurely.
Question 3. A certain codon is determined to be AUG.
- Of what nucleic acid molecule is this codon a part?
- What is the corresponding anticodon?
- Of what nucleic acid molecule is this anticodon a part?
- What is the deoxyribonucleotide responsible for this codon?
Answer:
- By definition, codon occurs only in mRNA.
- UAC
- By definition, anticodons occur only in tRNA
- TAC
Question 4. The alpha (α) polypeptide chain of human hemoglobin consists of 141 amino acid residues. Would you expect the DNA segment responsible for the ultimate synthesis of this chain to be shorter than, longer than, or about the same length as the functional mRNA molecule for this chain?
Answer: Longer
Question 5. An antibiotic is a microbial product of low molecular weight that specifically interferes with the growth of microorganisms when it is present in exceedingly small amounts. Specify some of the physiological activities that might be interrupted by an appropriate antibiotic and the reason why human cells are not harmed.
Answer:
- Cell wall formation is interfered with by penicillins and cephalosporins. The bleomycin and anthracyclines prevent DNA replication. Rifamycins interrupt the transcription of DNA into RNA.
- The translation is disrupted by erythromycin, tetracyclines, chloramphenicol, neomycin, lincomycin, puromycin, and streptomycin. Antibiotics are toxic for microorganisms but safe for humans because all of these metabolic processes are subtly different in bacteria and humans.
Question 11. Which processes in protein synthesis require hydrolysis of GTP?
Answer: Formation of the 70S initiation complex and translation.
Translation Protein Synthesis Multiple Choice Questions And Answers
Question 1. The ‘central dogma’ states that biological information flaws in which of these patterns?
- DNA → Protein → RNA
- RNA → DNA → Protein
- Protein -→ RNA → DNA
- DNA → RNA → Protein
Answer: 4. DNA → RNA → Protein
Question 2. One of these cannot be prepared directly from DNA
- Another DNA
- mRNA
- Protein
- tRNA
Answer: 3. Protein
Question 3. Amino acid sequence, in protein synthesis, is decided by the sequence of
- rRNA
- tRNA
- mRNA
- cRNA
Answer: 3. mRNA
Question 4. One of the following is not involved in the translation
- mRNA
- tRNA
- DNA
- rRNA
Answer: 3. DNA
Question 5. Codes of mRNA and amino acid residues of proteins are
- Colinear
- Coplanar
- Zig-zag
- None of these
Answer: 1. Colinear
Question 6. The name of Temin and Baltimore is associated with
- Photorespiration
- RNA synthesis
- Reverse transcription
- All of these
Answer: 3. Reverse transcription
Question 7. Enzymes involved in reverse transcription
- Primase
- Reverse transcriptase
- DNA polymerase
- Terminase
Answer: 2. Reverse transcriptase
Question 8. RNA retrovirus has a special enzyme that
- Polymerizes host DNA
- Disintegrates host DNA
- Translates host DNA
- Transcribes viral RNA to DNA
Answer: 4. Transcribes viral RNA to DNA
Question 9. The starting tRNA of prokaryotes is loaded with
- Valine
- Methionine
- Formylated methionine
- Tryptophan
Answer: 3. Formylated methionine
Question 10. The initiation of polypeptide chain formation is always brought about at the site of codon coding for an amino acid called
- Isoleucine
- Cysteine
- Phenylalanine
- Methionine
Answer: 4. Methionine
Question 11. Which of the following is associated with protein synthesis?
- Aminoacyl tRNA
- Initiation factors IF3, IF2, IF1
- Peptidyl transferase
- Transposons
Answer: 1. Aminoacyl tRNA
Question 12. The site of protein synthesis in a cell is
- Mitochondria
- Chloroplasts
- Ribosomes
- Plasma membrane
Answer: 3. Ribosomes
Question 13. During translation, initiation in prokaryotes, a GTP molecule is needed in
- Formation of formyl-met-tRNA
- Binding of 30S subunit of the ribosome with mRNA
- Association of 50S subunit of the ribosome with the initiation complex
- Association of 30S-mRNA with formyl-met-tRNA
Answer: 4. Association of 30S-mRNA with formyl-met-tRNA
Question 14. Which step of translation does not consume high-energy phosphate bonds?
- Translocation
- Amino acid activation
- Peptidyl transferase reaction
- Aminoacyl tRNA binding to A site
Answer: 4. Aminoacyl tRNA binding to A site
Question 15. Translocase is an enzyme required for
- DNA replication
- RNA synthesis
- Initiation of protein synthesis
- Chain elongation in protein synthesis
Answer: 4. Chain elongation in protein synthesis


Leave a Reply