Structure And Properties Of Metals And Alloys
All matter can be classified as either metals, nonmetals or metalloids.
A metal can be defined as a substance with high thermal and electrical conductivity, lustre, and malleability, which readily loses electrons to form positive ions (cations). They yield basic oxides and hydroxides. Roughly 80 percent of the periodic table are metals.
Metalloids are borderline elements showing both metallic and nonmetallic properties. Examples are carbon and silica.
Read And Learn More: Basic Dental Materials Notes
Uses in dentistry
Metals and their alloys are used extensively in dentistry. They are used in restorations, prostheses, etc. They are used in orthodontics in the form of wires and various appliances for the movement of teeth. Various instruments and appliances used in clinics and laboratory dentistry are fabricated from metals.
In dentistry, metals or their alloys are used in three capacities
- Dental restorations
- Direct intracoronal restorations, e.g. direct filling gold (DFG)
- Indirect (cast) coronal and extracoronal restorations like inlays and crowns, e.g. alloys of gold, platinum, chrome, cobalt, etc.
- Prosthetic uses, e.g. implants, intra and extraoral prostheses like dentures, and maxillofacial prostheses using cast alloy frames.
- Functional appliances and splints, e.g.
- Orthodontic therapy, e.g. wires and appliances for tooth movement
- Surgical uses, e.g. titanium plates
- Auxiliary uses, e.g. clinical and laboratory instruments and materials
Metallurgy
- Metallurgy is the study of the physical and chemical behavior of metallic elements, their intermetallic compounds, and their mixtures, which are called alloys.
Periodic table
- Dmitri Mendeleev was the first scientist to create a periodic table of elements (1869) similar to the one we use today. This table showed that when the elements were ordered by increasing atomic weight, a pattern appeared where the properties of the elements were repeated periodically. Thus the periodic table is a chart that groups the elements according to their similar properties.
- Features
- The periodic table helps predict some properties of the elements compared to each other. Atom size decreases as you move from left to right across the table and increases as you move down a column. The energy required to remove an electron from an atom increases as you move from left to right and decreases as you move down a column. The ability to form a chemical bond increases as you move from left to right and decreases as you move down a column
- There are 118 elements in the periodic table. Roughly 80 percent (94) of these elements are classified as metals.
- Groups
- Columns of elements help define element groups. Elements within a group share several common properties. Groups of elements have the same outer electron arrangement. The outer electrons are called valence electrons. Because they have the same number of valence electrons, elements in a group share similar chemical properties.
General Properties of Dental Metals
The general properties of Dental metals are
- Most metals are solid except for hydrogen which is a gas, and mercury and gallium which is liquid at room temperature.
- Good thermal and electrical conductivity.
- Hard, strong, and dense.
- Generally malleable and ductile, deforming under stress without cleaving.
- Optical properties, metals are shiny and lustrous.
- They make a metallic sound when struck.
- Forms positive ions in solutions during electrolysis.
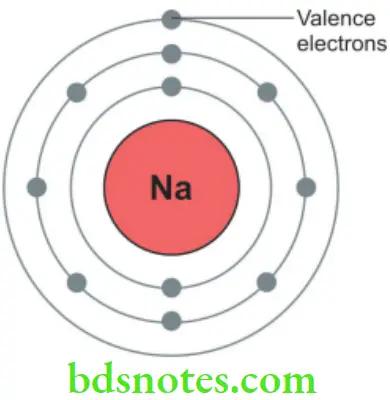
Dental Valence electron
- The outermost electrons of the atom are known as valence electrons (Fig. 3.2). Metals have outer valence electrons that are highly mobile. These are readily given up and are responsible for most of their properties.
- Valence electrons are an essential part of the atom’s stability. For the most part, eight valence electrons are necessary for an atom to be stable. A valence electron participates in the formation of a chemical bond with other elements.
Dental Metallic Bonding
- Metals are tough because atoms of the metals are held together by means of metallic bonds. Metallic bond is the bonding between molecules within metals (also called alkali reactive force).
- In metals, valence electrons are shared by all the atoms of the metal and so are not considered to be associated with any one atom. This is very different from ionic or covalent bonds, where electrons are held by one or two atoms. The sea of shared ‘delocalized electrons’ amongst a lattice of positive ions acts as a kind of “electron glue” giving the substance a definite structure.
- The metallic bond is, therefore, strong and uniform. Since electrons are attracted to many atoms, they have considerable mobility to be good conductors of heat and electricity.
- Metallic bonds, though strong, also allow the movement of individual atoms. This property allows metals to be hammered into sheets or drawn into wires.
Dental Alloys
- Most metals used in dentistry are used in the alloyed form. Examples of alloys in dentistry are steel, chrome-cobalt, nickel-chrome, gold-palladium, etc.
- One of the first alloys made by humans was bronze, which was made by mixing the metals tin and copper.
- Definition
- An alloy is a material composed of two more elements; at least one of which should be a metal. Thus an alloy is made by fusing two or more metals, or a metal and a nonmetal.
- An alloy may be a solid solution of the elements (a single phase), a mixture of metallic phases(two or more solutions), or an intermetallic compound with no distinct boundary between the phases.
- Solid solution alloys give a single solid phase microstructure, while partial solutions exhibit two or more phases that may or may not be homogeneous in distribution, depending on the thermal (heat treatment) history of the material. An inter-metallic compound has one other alloy or pure metal embedded within another pure metal.
- Alloys are used in some applications, where their properties are superior to those of the pure component elements for a given application.
- Why do we need to alloy?
- An alloy has properties that are beneficial over that of the parent materials. A metal that is normally very soft and malleable, such as aluminum, can be altered by alloying it with another soft metal, like copper. Although both metals are very soft and ductile, the resulting aluminum alloy will be much harder and stronger. Adding a small amount of non-metallic carbon to iron produces an alloy called steel. Due to its very high strength and toughness (which is much higher than pure iron), and its ability to be greatly altered by heat treatment, steel is one of the most common alloys in modern use. By adding chromium to steel, its corrosion resistance can be enhanced, creating stainless steel, while adding silicon will alter its electrical characteristics, producing silicon steel.
- Classification of alloys
- Alloys are usually classified as substitutional or interstitial alloys, depending on the atomic arrangement that forms the alloy.
- They can further be classified based on solubility as
- Homogeneous (consisting of a single phase)
- Heterogeneous (consisting of two or more phases)
- Intermetallic (where there is no distinct boundary between phases)
- During the fabrication of a restoration, the alloy is melted and the liquid metal is forced into a mold to create the particular restoration. Thus a knowledge of the behavior of alloys during melting and solidification is beneficial.
- Manufacture of alloys
- Alloys may be prepared by different technological methods: melting, sintering of a powder mixture, high-temperature diffusion of an alloying element into the base metal, plasma and vapor deposition of different elements, electroplating, etc.
Phase
- A phase is a uniform part of an alloy, having a certain chemical composition and structure, and which is separated from other alloy constituents by a phase boundary.
- An alloy phase may be in the form of a compound (substance formed from two or more elements), with a fixed ratio determining the composition) or in the form of a solid solution.
Solid Solutions
Can one metal dissolve in another?
- Two or more metals can dissolve to form solutions. This usually occurs at high temperatures and the product formed is called an alloy. Some metals can also be dissolved at room temperature, e.g. mercury-tin. This property is used in the creation of dental amalgam.
Solid solution
- A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by the addition of the solutes, and when the mixture remains in a single homogeneous phase. This often happens when the two elements (generally metals) involved are close together on the periodic table; conversely, a chemical compound generally results when two metals involved are not near each other on the periodic table.
- The solid solution needs to be distinguished from a mechanical mixture of powdered solids like two salts, sugar and salt, etc. The mechanical mixtures have a total or partial miscibility gap in the solid state.
- Examples of solid solutions include crystallized salts from their liquid mixture, metal alloys, and moist solids. In the case of metal alloys intermetallic compounds occur frequently.

Types of solid solutions
Depending on the ratio of the solvent (matrix) metal atom size and solute element atom size, two types of solid solutions may be formed.
- Substitutional
- Interstitial
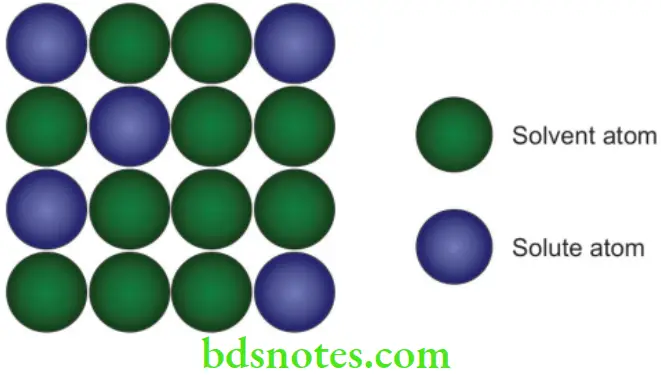
Substitution solid solution
- If the atoms of the solvent metal and solute element are of similar sizes (not more than 15% difference), they form a substitution solid solution, where part of the solvent atoms are substituted by atoms of the alloying element.
- Examples are brass (copper with zinc) and palladium-silver.
Interstitial solid solution
- If the atoms of the alloying elements are considerably smaller than the atoms of the matrix metal, interstitial solid solution forms, where the matrix solute atoms are located in the spaces between large solvent atoms (Fig. 3.4).
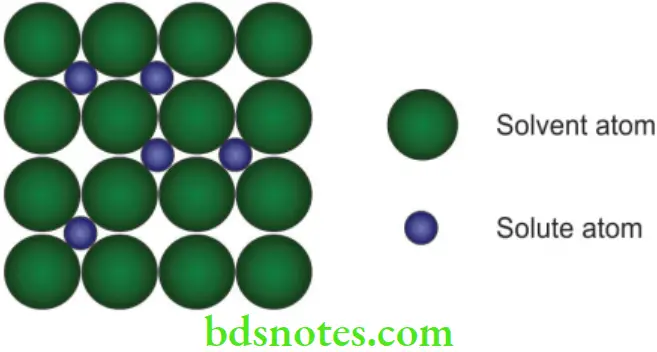
Steel is an example of an interstitial alloy in which a relatively small number of carbon atoms slip in the gaps between the huge iron atoms in a crystalline lattice of iron. Titanium (CPTi) used in dental implants is another example (99% titanium with oxygen, carbon, nitrogen, and hydrogen as interstitial substitutes).
Dental Applications
- Most noble alloys used in dentistry are based on solid solutions. In the case of Pd-Ag, both metals are mutually soluble in each other in the solid state regardless of the proportion of each.
Solidification and microstructure of metals and alloys
- Microstructure is defined as the structure of a prepared surface or thin foil of material as revealed by a microscope. The microstructure of a material can strongly influence physical properties such as strength, toughness, ductility, hardness, corrosion resistance, high/low-temperature behavior, wear resistance,
- And so on, which in turn, govern the application of these materials in laboratory and clinical practice. Microstructure at scales smaller than can be viewed with optical microscopes is often called ultrastructure or nanostructure.
Grains
- Solidification occurs by a process of crystallization. Crystallization begins around a nucleus. Crystals are also known as grains as they seldom show the customary crystal shapes because of interference from neighboring crystals. The crystallization process results in various types of microstructures depending on the type of metal or alloy and the cooling process.
- The crystals are otherwise known as grains since they seldom exhibit the customary geometric forms due to interference from adjacent crystals during the change of state.
Nuclei of crystallization
- The surface tension of liquid metal is 10 times higher than that of water. When molten alloy cools, the solidification process begins with the formation of atom clusters called ‘embryos’ or ‘nuclei of crystallization’.
Crystallization forms
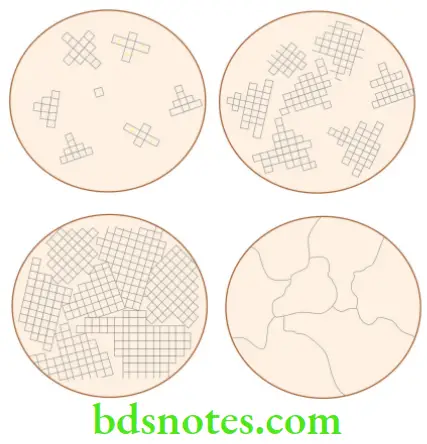
Metals solidify in roughly three predominant crystal forms
- Dendritic microstructure
- Polycrystalline microstructure
- Equiaxed grains
- Columnar grains
- Monocrystalline or Single-crystal
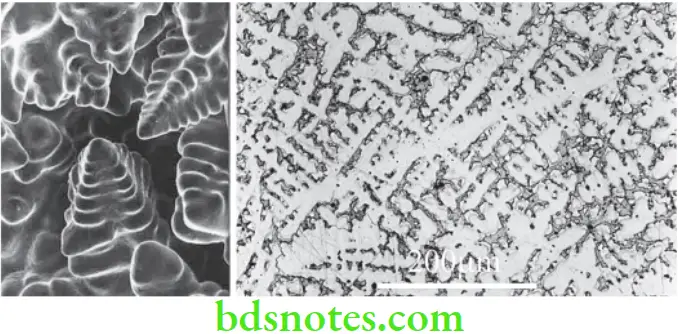
Dendritic microstructure
- In the dendritic form, the crystals that form in the liquid during freezing generally follow a pattern consisting of a main branch with many appendages. A crystal with this morphology slightly resembles a pine tree and is called a dendrite, which means branching. The formation of dendrites occurs because crystals grow in defined planes due to the crystal lattice they create. Secondary dendrite arms branch off the primary arm, and tertiary arms off the secondary arms, etc., resulting in a three-dimensional dendritic structure.
- Dental base metal alloys and titanium-based casting alloys in which nickel (N), cobalt (Co), iron (Fe), and titanium (Ti) are the main elements generally have a dendritic as cast microstructure.
Equiaxed polycrystalline microstructure
- Equiaxed grains are crystals that have axes of approximately the same length. The crystal grows from multiple nucleation points. The expanding crystals continue to grow until they impinge on adjacent growing crystals at grain boundaries. The final sizes of the individual crystals depend on the number of nucleation points. Most noble metal casting alloys solidify with this type of microstructure.
- Dental noble metal alloys generally have equiaxed fine-grain microstructures because of certain grain refining elements added into the composition.
Columnar polycrystalline alloys
- Columnar grain structured castings are created using a special casting technique called ‘directional solidification’. They have grains parallel to the major stress axes. They are less prone to creep deformation compared to equiaxed grain structured metal components.
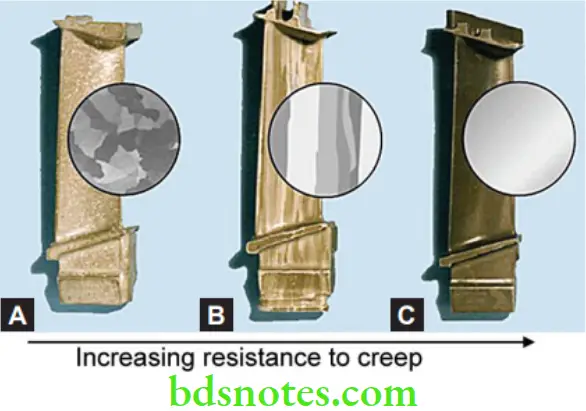
Single crystal
- Normally when a material begins to solidify, multiple crystals begin to grow in the liquid, and a polycrystalline (more than one crystal) solid forms. However, it is possible to produce a single large crystal, so there are no grain boundaries in the material.
- Single crystals are produced under carefully controlled conditions. The expense of producing single-crystal materials is only justified for special applications, such as turbine engine blades, solar cells, and piezoelectric materials.
- Jet engine turbine blades must endure tremendous forces at extremely high temperatures for prolonged periods. Under such conditions, metals with a grain structure tend to “creep,” or slowly deform, along grain boundaries. Because single-crystal alloy parts have no grain boundaries they are highly resistant to creep.
- Forming single-crystal metal objects requires both special alloys and special casting techniques (a modified version of the directional solidification technique). The alloys are almost always nickel-based, with as many as nine minor metal components including chromium, cobalt, tungsten, tantalum, aluminum, and/or rhenium.
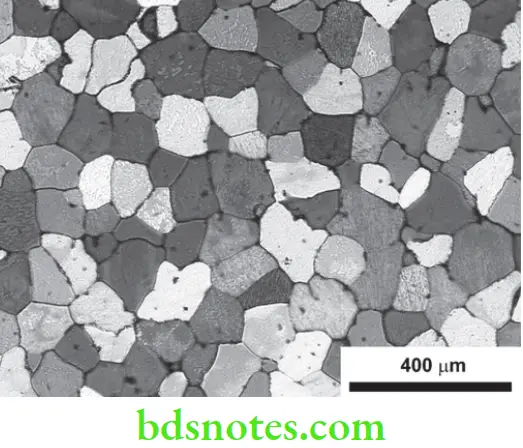

Grain Boundaries
As mentioned previously, the grains meet at grain boundaries (Fig. 3.8) which are regions of transition between differently oriented crystals.
These are regions of importance
- Less resistance to corrosion
- High internal energy
- Collection of impurities
- Barriers for dislocations
Grain refinement and Grain size
Control of grain morphology and size is important in order to predict the properties of the cast metal. Generally, alloys with fine grains have superior properties including corrosion resistance.
Control of Grain Size
Grain size can be controlled by
- Addition of small quantities of grain refining elements like iridium (Ir), ruthenium ((Ru), rhenium ((Re), etc.
- Use of different casting techniques.
- Heat treatment, namely annealing.
Time-temperature graph
- To understand the solidification of alloys, one has to understand the solidification of a pure metal. This can be understood by melting a pure metal and then cooling it back to room temperature. As it cools, a time-temperature graph is plotted.
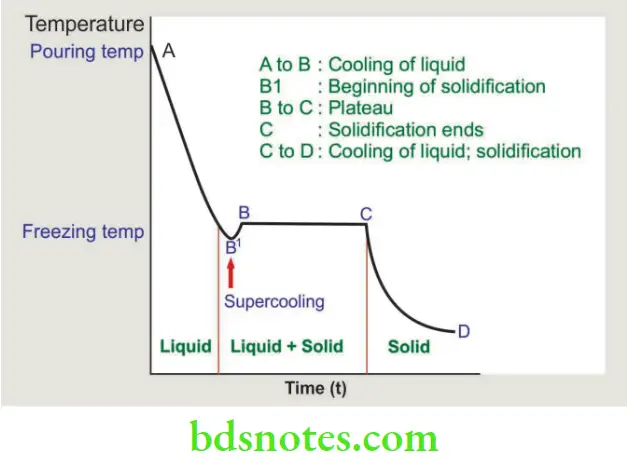
- The molten metal starts cooling from A to point B1, this is called supercooling. During the supercooling process crystals of metal begin to form. Crystallization releases latent heat of fusion causing a rise in temperature (B1 to B).
- Completion of crystallization occurs at point C. Between B and C, the metal has both liquid and solid phases. The temperature remains steady (plateau) until crystallization is completed. Point D indicates cooling to room temperature.
Equilibrium Phase Diagrams
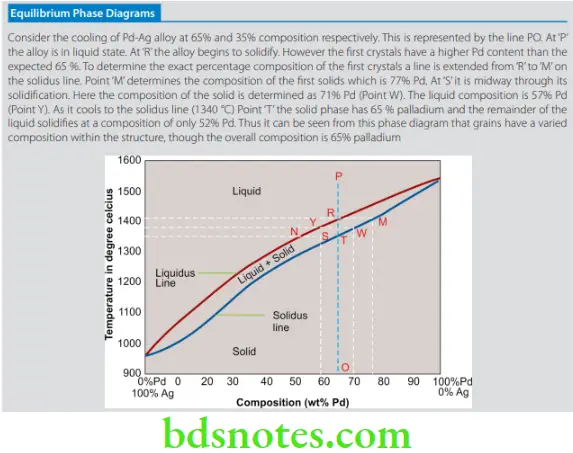
- A phase diagram is also known as a constitutional diagram. It helps to identify the phases present in an alloy at different compositions and temperatures. The phase diagram may be explained using the example of the palladium-silver alloy. The two metals exhibit complete solubility in liquid and solid states. The equilibrium phase diagram of Pd-Ag at a composition of 65% Pd and 35% Ag is presented.
Homogenization Heat Treatment – microstructure
- From the phase diagram of the palladium-silver alloy, it is evident that the composition of the structure is not uniform. The grains that form early on during solidification have a higher palladium content compared to the layers that solidify later. Thus the core contains dendrites of varying or nonuniform composition.
- Heat treatments can be used to homogenize cast metal alloys to improve their hot workability, to soften metals before, and during hot and cold processing operations, or to alter their microstructure in such a way as to achieve the desired mechanical properties.
Definition
- Homogenization heat treatment can be defined as a combination of heating and cooling operations applied to a metal or alloy in its solid state to eliminate as cast nonuniform compositional variations (coring effect) to a more uniform equiaxed grain structure through atomic diffusion.
Procedure
- The heat treatment of metals involves raising the temperature of an alloy to a defined temperature, usually near its solidus. The material is then held at this temperature for a period of time (up to 6 hours) before being cooled either at a prescribed rate or under rapid quenching conditions to a fixed temperature. Heat treatment is carried out in furnaces and ovens.
Eutectic Alloys
Eutectic is a ‘term’ used to describe two components that are ‘completely soluble’ in each other in the liquid or molten state, but only partially soluble in the solid state. Therefore these alloys do not form solid solutions. Rather, eutectic alloys upon cooling, convert from liquids to intimately mixed solids.
In an eutectic system, the “eutectic alloy” composition has the lowest melting point in the system. It is lower than the melting points of either of the pure components.
Applications
- Pb-Sn alloy is a good example of an eutectic alloy system.
- In Dentistry, the Au-Cu eutectic alloy is used in the silver amalgam system.
- Gold-iridium system is eutectic at an iridium concentration of 0.005%.
Equilibrium-phase diagrams of Ag-Cu
In the phase diagram, at three different concentrations, the material will be solid until it is heated to its melting point, and then (after adding the heat of fusion) becomes liquid at that same temperature.
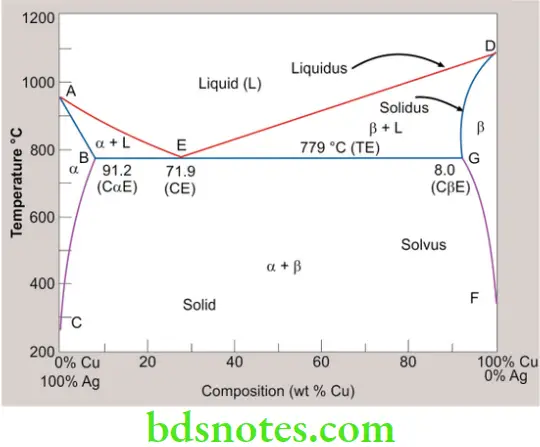
α – Silver-rich substitutional solid solution
β – Copper-rich substitutional solid solution
L – Liquid phase
AED – Liquidus line
ABEGD – Solidus line
Solidus and Liquidus lines meet at E rather than at the pure metal composition seen in the earlier phase diagram. This composition of 72% Ag and 28% Cu is called as the Eutectic.
Eutectic means lowest melting. The Eutectic composition melts at a temperature of 779 °C which is lower than either of the pure parent metals.
- The unalloyed extreme left
- The unalloyed extreme right
- The dip in the center E (the eutectic composition).
At other compositions, the material will enter a mushy or pasty phase until it warms up to its melting temperature.
The mixture at the dip point (E) of the diagram is called an eutectic alloy.
Features of the eutectic system
- The eutectic composition melts at a temperature of 779 °C which is lower than either of the pure parent metals. This property is useful in solders.
- Solidus and liquidus lines meet at the Eutectic temperature. Thus there is no solidification range at the eutectic temperature of 779 °C.
Hypoeutectic and hyper eutectic alloys
- Alloys with compositions less than that of the eutectic are called hypoeutectic alloys and those with a composition greater than the eutectic are known as hypereutectic alloys.
Peritectic Alloys
- Peritectic transformations are also similar to eutectic reactions. Here, a liquid and solid phase of fixed proportions react at a fixed temperature to yield a single solid phase.
- The peritectic reaction during the cooling phase can be written as
- Liquid + β Solid solution → α Solid solution
- Peritectic alloys are susceptible to coring during rapid cooling. This cored structure is more brittle and has lower corrosion resistance than a homogeneous solid solution.
- Examples
- The dental amalgam alloy silver-tin is based on the peritectic system.
- Dental casting alloy gold-platinum.
- Palladium-ruthenium alloy at 16.5% has a peritectic reaction.
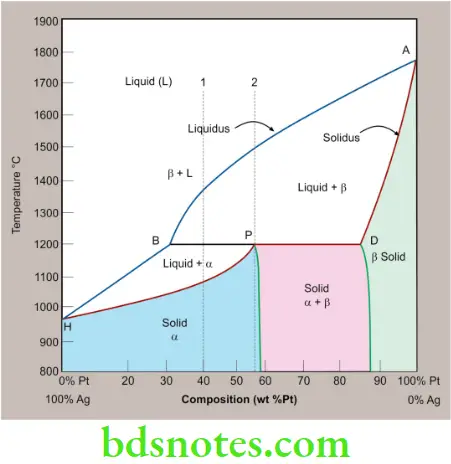
α – Silver-rich phase
β – Platinum-rich phase
P – Peritectic transformation point
A – Platinum melting point (1768 °C)
H – Silver melting point (961.8 °C)
A peritectic reaction is a reaction where a solid phase (β) and liquid phase will together form a second solid phase (α) at a particular temperature and composition. In this case, it occurs at a composition of 56% Pt.
Solid state reactions
- Knowledge of solid-state reactions is important to understand strengthening mechanisms in Type 3 and 4 gold alloys like age hardening. Solid-state reactions can be explained through the gold-copper phase diagram.
Gold-Copper phase diagram
- Gold and copper are completely soluble in each other in the liquid state. The gold-copper equilibrium phase diagram is presented in (Fig. 3.14). The difference between the liquidus and solidus is small in all compositions. The two curves meet at 80.1%.
- This composition marks the lowest temperature at which a gold-copper alloy can melt. At temperatures between the solidus and 410°C the gold and copper are mutually soluble and form a disordered solid solution (α).
- The atoms are in a random arrangement in an FCC structure. However, as the alloy cools down further, certain solid-state transformations are seen. The various phases of cooling at various compositions are summed.


Heat treatment of Gold alloys
- The ordered phases (α’, α’’2, and α’’1) have superior mechanical properties. The disordered phase (α phase) on the other hand is relatively soft and ductile. This soft ductile phase is convenient for laboratory finishing and grinding procedures. The disordered phase which exists at higher temperatures can be preserved by rapid quenching. Rapid cooling prevents atomic movement to the more ordered lower temperature phases. Following adjustments of the crown, the alloy is again age-hardened by holding it at a specified temperature for a specified period. This permits the solid state reactions to take place (through atomic fusion) and convert the alloy to its mechanically superior ordered phases (α’, α’’2, and α’’1). The alloys can be restored to their softened condition by again raising it to a temperature just below its solidus (700°C) and holding it at this temperature for 10 minutes and then rapidly quenching. Thus essentially, gold alloys can be both softened and hardened through heat treatment.
Types of Heat Treatment
- From the above, it is clear that a variety of heat treatments are available for various purposes. The various heat treatments are summarized below.
Homogenization
- This treatment is used before hot working processes and is performed to equalize temperatures throughout an alloy or to reduce the coring effect caused by the nonuniform chemical composition.
Softening heat treatment
- Synonyms Solution heat treatment, annealing.
- Softening heat treatment covers a variety of heat treatment processes used to soften alloys and increase their ductility as an aid to cold working. It is also performed to remove internal stresses within components following cold working, welding, casting, or rapid cooling.
Hardening heat treatment
- Synonyms Precipitation hardening, age hardening
- Hardening heat treatment is a heat treatment technique used to increase the strength of malleable materials, including most structural alloys of nickel, titanium, and some stainless steel.
Classification of alloys
Dental alloys can be classified in many ways.
- Based on the number of alloying elements
- Binary (two constituents)
- Ternary (three constituents)
- Quaternary (four constituents)
- Quinary (five constituents)
- Based on noble metal content
- High Noble
- Noble
- Predominantly base
Functions of alloying elements
- Elements are added to the alloy in various proportions to modify its properties. Most elements affect the properties of the metal in multiple ways. The various properties and the effect of the modifiers are described.
Grain refinement
Grain refinement is the process by which the grain size is restricted during solidification. Smaller grain sizes block dislocation movements within the structure. This improves the yield strength of the alloy.
Metals that refine grain sizes are
- Iridium
- Rhenium
- Ruthenium
Mechanical properties
- Alloys like palladium, platinum, copper, and iron improve mechanical properties like strength, hardness, and modulus of elasticity in gold-based alloys.
Color modification
- The alloying metals have varying effects on the color of the alloy. Metals like gold and copper impart a gold and reddish hue to the alloy. White metals like palladium and silver tend to whiten the alloy. Silver-rich alloys tend to have a green hue.
CTE (TEC) modification
- The thermal expansion coefficient (TEC) (also called the coefficient of thermal expansion CTE) of an alloy used to construct porcelain-fused to metal crowns (PFM) requires to be closely matched to that of the veneering porcelain. TEC mismatch can cause fracture or separation of the veneer from the metal. Silver increases CTE whereas palladium lowers the CTE in the alloy.
Melting range modification
- Generally, metals with higher melting points raise the melting range, whereas metals with lower melting points tend to lower the melting range of the corresponding alloys. For example, silver reduces the melting range of palladium and gold alloys. Palladium and platinum raise the melting range of gold-based alloys.
Castability
- The castability of a material is the ease with which a casting can be produced with minimal energy and defects. Generally, castability is related to the density of a material. Denser metals improve the castability of the alloy. Noble alloys are generally more easy to cast because of their greater density when compared to base metal alloys like cobalt-chromium.
Oxygen scavenger
- Zinc is added to remove oxygen during the casting process, thereby reducing porosity.
Tarnish and corrosion resistance
- Tarnish and corrosion resistance is a desirable property, especially in the oral environment. Improving the concentration of noble metals improves the tarnish and corrosion resistance of an alloy.
- In base metal alloys, the mechanism for tarnish and corrosion resistance is different. In chrome-based alloys, a surface oxide is readily formed. The surface oxide forms a protective layer that protects the metal from further corrosion. This property is called ‘passivation’. Other metals which use a similar mechanism are stainless steel and titanium.
Density and specific gravity
- In certain instances like large cast removable partial dentures, a lightweight framework is preferable to a more heavier frame in terms of patient comfort and appliance retention. Base metal alloys are popular as RPD alloys because of their low density. Palladium is lighter than gold lowering the density and weight of gold-palladium-based alloys. The density of a palladium-based alloy is midway between that of base metal and of high noble alloys.
Oxide formation
- Metals used for porcelain fused to metal restorations have the additional requirement of facilitating porcelain attachment. Porcelain does not bond readily to noble metal alloys. Certain elements like tin, indium, and iron oxide are added to noble metal alloys to form oxides which aid in the bonding of porcelain to the alloy. Base metal alloys form oxides quickly.

Leave a Reply