Sterilization And Disinfection
Question 1. Describe sterilization and methods of sterilization in brief.
Or
Define sterilization and in brief different methods of sterilization.
Or
Describe in detail different methods of sterilization.
Or
Write a short note on methods of chemical sterilization.
Or
Describe the physical methods of sterilization.
Or
Write a short note on physical methods of sterilization.
Or
Answer:
Sterilization is a process by which articles are freed of all microorganisms both in vegetative and spore states. Various methods of sterilization
There are two methods— physical and chemical:
Sterilization by Physical Methods:
1. Sunlight:
- It produces bactericidal activities. Its action is due to UV rays
- Sunlight is the natural method of sterilization that occurs under natural conditions.
2. Drying:
- Drying in the air has a deleterious effect on many bacteria.
- It is an unreliable method and spores are remain unaffected.
3. Heat:
- This is a reliable method of sterilization.
- Heat is used to sterilize the materials which do not get damaged by heat.
- Sterilization by heat is of two types, i.e. dry heat and moist heat.
4. Dry Heat:
Dry heat sterilization should be preferred for the sterilization of glass syringes, test tubes, Petri dishes, and materials such as oils, jelly, and powder. As compared to moist heat sterilization, dry heat sterilization is less efficient and needs a long time and high temperature for sterilization. The penetration power of dry heat is low, so it is not effective when non-conducting materials protect microorganisms.
Read And Learn More: Microbiology Question And Answers
Killing by dry heat is due to:
- Protein denaturation
- Oxidative damage
- Toxic effect of elevated levels of electrolytes.
Following are the procedures by which dry heat can be used:
- Red heat: In this method direct heating of an instrument or object in a flame is done till it becomes red hot. The method has limited applications. It is used for sterilizing non-inflammable materials. For example, inoculating wires or loops, needles, forceps, scissors, spatula, etc.
- Flaming: The article is passed over flame without allowing it to become red hot. Its efficacy is not certain. It is used to sterilize the mouth of cultured tubes, cotton wool plugs, and glass slides.
- Incineration: In this method, direct burning of material should be carried out at high temperatures, i.e. 800 to 1000°C. This is an excellent method for sterilization and rapidly destroying contaminated materials by direct burning. It is used for destroying materials,
For example: Soiled dressing bedding and pathological materials, etc. - Hot air oven: This method is the most common and widely used method of sterilization by dry heat. Sterilization by hot air oven requires a temperature of 160°C for one hour. We can sterilize all-glass syringes, Petri dishes, test tubes, pipettes, cotton swabs, scalpels, scissors, etc.
5. Moist Heat:
- Moist heat is very efficient in penetrating the material as compared to dry heat.
- Mainly this is used to sterilize culture media before washing, glass syringes, surgical instruments, laboratory coat, aprons, etc.
- The lethal effect of moist heat is by denaturation and coagulation of proteins.
- Moist heat should be given in the following ways, i.e.
- Temperature below 100°C
- Temperature around 100°C
- Temperature above 100°C.
Temperature below 100°C:
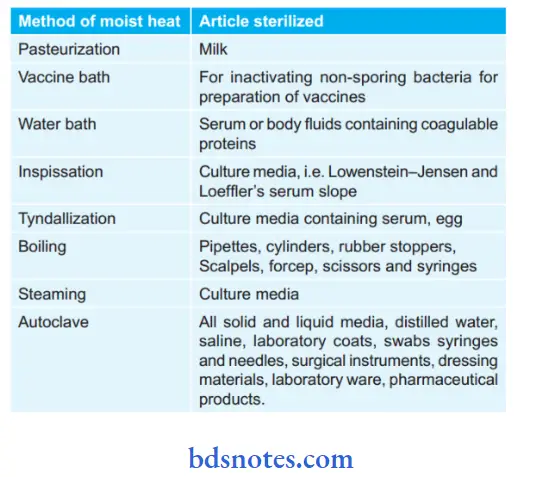
- Pasteurization of milk: Pasteurization is the method which is used for sterilization of the milk. The temperature employed is either 63°C for 30 minutes (Holder method) or 72°C for 15 to 20 seconds followed by cooling quickly to 13°C or lower (flash method). Organisms like Mycobacterium, Salmonella, and Brucellae are killed. Only Coxiella burnetii survive the holder’s method which is resistant to it. Spores may not be destroyed.
- Vaccine bath: It is used for killing non-sporing bacteria which may be present in the vaccine. In this method heating is done for 1 hour at 60°C in a vaccine bath.
- Water bath: This is used for the sterilization of serum or body fluids which consists of coagulable protein. In this method, heating is done for 1 hour at 56°C for several successive days in a water bath.
- Inspissation: This method is used for the sterilization of, Lowenstein–Jensen medium and Loeffl’s serum slope. In this method, heating is done at 80°C for half an hour on three successive days in an inspissator.
- Principle of Inspissator:
- On the first day during first exposure all vegetative forms are killed.
- On the first day spores that do not get killed, germinate to form vegetative forms before the second exposure.
- On the second day as exposure is done newly formed vegetative forms get killed.
- If still spores are present, germinate till the third exposure, and as the third exposure is given on the third day complete sterilization is ensured.
Temperature around 100°C:
- Tyndallization: It is the fractional sterilization method. This is the process by which the medium is placed at 100°C in flowing steam for 30 minutes on three successive days. The mechanism underlying this method is that vegetative cells get destroyed at 100°C. Tyndallization is applied only to nutrient media.
- Principle: One first exposure vegetative forms are killed. Spores which are present germinate on successive days and are killed by the second or third exposure.
- Boiling: Most of the vegetative form of bacteria is killed at 100°C for 10 to 30 minutes. But this does not ensure complete sterilization as some spore-forming bacteria may not be eliminated completely. In this method addition of 2% sodium carbonate promotes sterilization.
This method is recommended for the sterilization of rubber stopers, cylinders, scissors, pipettes, scalpel, syringes, etc. This method should not be used to sterilize the instruments which are used in the surgical procedures. - Steam at atmospheric pressure: Here free steam is used to sterilize culture media which consists of sugar and gelatin, which may decompose if subjected to a higher temperature. One exposure for 90 minutes provides surety of complete sterilization. Koch or Arnold steam sterilizer is used.
Temperature above 100°C:
- This is the most widely used method for sterilization.
- Sterilization is carried out at a temperature between 108°C to 147°C Sterilization is done by generating steam.
- Autoclave is the most commonly used sterilizer.
6. Autoclave:
Principle: Water boils when its vapor pressure equals that of the surrounding atmosphere.
- Boiling of water occurs at 100°C when its vapor pressure is equal to atmospheric pressure but when pressure inside a close vessel increases the temperature at which water boils also increases.
- Saturated steam has good penetrating power and microbes are susceptible to moist heat.
- When steam comes in contact with a cooler surface it condenses to water and gives latent heat to that surface.
- Reduction in volume sucks more steam to the area and the process continues till the temperature of that surface is raised to that of steam.
- Condensed water ensures moist conditions for killing microbes present.
Sterilization Cycles of An Autoclave:

7. Ozone:
Ozone sterilizer utilizes oxygen, water, and electricity to produce ozone in the sterilizer and provides sterilization and does not produce any toxic chemicals. Ozone Runs at Lower Temperatures, i.e. 25°C to 35°C.
8. Mechanism of action:
The oxygen molecules are separated into atomic oxygen in the presence of an intense electrical field. This atomic oxygen combines with other oxygen molecules and forms ozone which provides sterility assurance of 10-6 in 4 hours.
9. Radiation:
- Microorganisms absorb the radiant energy which is why radiation has an effect on microorganisms.
- Two types of radiation is used to sterilize the instruments, i.e. non-ionizing radiation and ionizing radiation.
- Non-ionizing Radiation:
- Ultraviolet rays constitute non-ionizing radiation.
- Ultraviolet rays are short rays with low penetrating power.
- The effective wavelength of ultraviolet rays is between 240 nm and 280 nm. 254 nm is considered to be the most effective wavelength for sterilization.
- Ultraviolet rays are mutagenic and produce lethal photochemical effects on enzymes and cell constituents, i.e. protein, DNA, etc.
- Ultraviolet rays kill cells, delay cell division and as well as synthesize of certain substances by cell.
- In anaerobic conditions, it is most effective.
- All the microorganisms including spores are sensitive to ultraviolet rays treatment. Spores need double exposure.
- It is used for air disinfection in entryways, hospital wards, operating rooms, and laboratories; for disinfecting drinking water; for preparation of bacterial and viral vaccines.
- Ionizing Radiation:
- It consists of gamma rays, beta rays, X-rays, etc.
- Above mentioned rays have a very high penetrating power.
- Ionizing radiation is highly lethal to DNA as well as to other viral contents.
- This is also known as cold sterilization as there is no increase in the temperature.
- Mainly gamma and beta rays are used for sterilization of instruments and dressing packs, i.e. plastic syringes, swabs, culture plates, fabrics, metal foils, and catheters.
- X-rays are not used for sterilization as they have poor penetrating power and they also induce radioactivity in exposed materials.
10. Filtration:
- Filtration is mainly used to sterilize thermolabile substances.
- In the filtration process, filters are used for removing the microorganisms from liquids that are thermolabile.
- It is done under negative pressure.
- In infiltration, fluid is sucked via a filter in a receiving flask which is connected to the exhaust pump. The exhaust pump leads to the suction of fluid through the fitter. It is used in sterilizing medicines or other materials which are heat labile; for purifying water; To separate and study microorganisms.
- Various examples of filters used in the filtration process are Candle filters, asbestos filters, sintered glass filters, and membrane filters.
Methods of Chemical Sterilization
Different varieties of chemical agents are used as antiseptics and disinfectants.
Phenols:
- Phenols are the most commonly used disinfectants.
- Based on their concentration phenols are both bactericidal and bacteriostatic.
- Phenol compounds are carbolic acid, cresol, and halogenated diphenyl compounds.
Carbolic Acid:
- 1% phenol is bactericidal, it damages the cell membrane and causes its lysis.
- This is effective for vegetative forms of bacteria, mycobacterium tuberculosis, and various fungi.
- It is used as a disinfectant for feces, pus, blood, etc.
Cresol:
- Due to low solubility in water phenols are formulated with soaps, which increases the bactericidal activity of phenols.
- An example is Lysol which is the solution of cresol in soap.
- Cresol is more germicidal as well as less poisonous as compared to other phenols.
- It can be corrosive to living tissue so long-term contact is avoided.
- Uses: For disinfecting surgical instruments, glassware, excreta, furniture, and other contaminated objects.
Halogenated Diphenyl Compounds:
- These are hexachlorophene and chlorhexidine
- Hexachlorophene should be used with caution as it is toxic.
- Chlorhexidine is effective both for gram-positive and gram-negative microorganisms. This is used as a skin antiseptic and for the treatment of wounds.
Ethanol:
- Mainly ethyl alcohol and isopropyl alcohol are employed as skin antiseptic agents.
- The action of alcohol is by denaturing bacterial proteins and also by disorganizing the lipid structure of the cell membrane.
- These both are active at concentrations of 60% to 70% in water.
- Alcohols are not effective against spores and viruses.
- Isopropyl alcohol is preferred to ethyl alcohol as this is a better fat solvent, more bactericidal, and less volatile.
- It is used for the disinfection of clinical thermometers.
- Methyl alcohol is effective against fungal spores and is used for disinfecting cabinets.
Oxidizing Agents:
It consists of halogens, hydrogen peroxide, potassium permanganate, sodium perborate, etc.
- Halogens:
- Halogens are chlorine and iodine.
- Chlorine and its compounds are used for the disinfection of water, dairy equipment, and sanitary, utensils in the food industry.
- Sodium hypochlorite is used for air disinfection. Organic chloramines are used as antiseptics for dressing of wounds.
- Iodine is used as a skin disinfectant. It is used as a tincture, i.e. alcoholic solution consisting 2.5% iodine + 2.5% potassium iodide in 90% of alcohol.
- Iodophores, i.e. iodine combined with surface active agents is generally used and is claimed to be more effective than a tincture.
- Hydrogen Peroxide: It is used at a concentration of 3% solution.
- It is used to clean wounds and as mouthwash or gargle.
- Potassium Permagnate:
- This is bactericidal in nature and is also active against viruses.
- This is used in the treatment of urethritis.
Acids and Alkalis:
- Various acids such as sulphuric acid, nitric acid, hydrochloric acid, benzoic acid, etc., and alkalis like potassium and sodium hydroxide and ammonium hydroxide are germicidal in nature.
- By hydrolysis and altering the pH of the medium they kill microorganisms.
- These are rarely used as disinfectants.
Dyes:
- Dyes used to stain bacteria are bacteriostatic in nature.
- Crystal violet, malachite green, brilliant green, etc. are active against gram-positive organisms but have less effect on gram-negative organisms
- They are used as skin and wound antiseptics.
Heavy Metals:
- Heavy metals are the soluble salts of mercury, silver, copper, arsenic, and other heavy metals that have antibacterial activity—bactericidal and bacteriostatic activity.
- Mercuric chloride act as a disinfectant
- Organic compounds, i.e. merthiolate and mercurochrome act as antiseptics.
- Silver compounds act as antiseptics while organic silver salts are bactericidal agents.
- Arsenic compounds are used in the treatment of syphilis.
Alkylating Agents:
- Formaldehyde, glutaraldehyde, and ethylene oxide act as alkylating agents.
- These exert lethal effects on proteins.
- Formaldehyde:
- Formaldehyde is bactericidal, and sporocidal, and is effective against viruses in its aqueous solution.
- Formalin which is an aqueous solution of 37% formaldehyde is used as a coagulant and preservant for destroying anthrax spores in hair and wool.
- Formalin gas is used for fumigation of operation theaters, wards, sick rooms, and laboratories, as sterilization of instruments, and heat-sensitive catheters.
- For fumigating a room of 28.3 m3, 150 g of potassium permanganate should be added to 280 ml of formalin, and fumigation is done after closing the windows and other outlets to generate the gas.
- All the doors and windows are closed and sealed to 48 hours.
- Ethylene Oxide:
- This is an alkylating agent which is to be used in gaseous sterilization.
- Ethylene oxide is active for all bacteria, spores, and viruses.
- It is used to sterilize any object but mainly it is used to sterilize the objects which get damaged by heat.
- For example, sutures, dental equipment, etc.
- Gluteraldehyde:
- This is active mainly against tubercle bacilli, fungi, and viruses.
- This is used as a buffered solution for cleaning cystoscopes and bronchoscopes, face masks, metal instruments, etc.
Beta-propiolactone:
- This is the condensation product of formaldehyde and ketone having a boiling point of 163°C.
- Its penetrating power is low but more efficient in fumigation as compared to formaldehyde.
- This is active for all microorganisms and is more active against viruses.
0.2% of beta-propiolactone is used for the sterilization of biological products.
Surface Active Agents:
- Surface active agents are those which alter energy relationship at interfaces and decreases interfacial tension.
- They are widely used as detergents, wetting agents, and emulsifiers.
The following is the type of surface active agents:
- Cationic Surface Active Agents:
- These agents consist of quaternary ammonium compounds which are more active at alkaline pH. Examples are: Cetyltrimethylammonium bromide and benzalkonium chloride.
- These agents produce their action by denaturing proteins.
- They are bactericidal and are more active for gram-positive bacteria.
- They are not active for spores, tubercle bacilli, and viruses.
- Anionic Surface Active Agents:
- These agents are soaps that better act at acidic pH.
- Soaps are both prepared from saturated and unsaturated fatty acids.
- Those soaps which are prepared from saturated fatty acids, i.e. coconut oil and are more effective against gram-negative bacteria while those soaps which are prepared from unsaturated fatty acids, i.e. oleic acid are more effective against gram-positive bacteria
- Non-Ionic Surface-Active Agents:
- These are non–toxic.
- Some of these promote the growth of bacteria, for example 80 produces the growth of tubercle bacilli.
- Amphoteric or Ampholytic Compounds:
- They are active against both Gram-negative bacteria and Gram-positive bacteria and some of viruses.
- These are called as tego compounds and are not in use.
Question 2. Define sterilization and disinfectant. Enumerate various methods of sterilization.
or
Describe sterilization by methods using moist heat and name articles sterilized by moist heat.
Or
Define and classify sterilization. Add a note on the moist heat method of sterilization.
Or
Write briefly methods of sterilization of moist heat.
or
Write a short note on moist heat sterilization methods.
Or
Define and classify methods of sterilization. Write in brief methods of utilizing moist heat.
Answer:
Sterilization definition:
Sterilization is a process by which articles are freed of all microorganisms both in vegetative and spore states. Disinfection is a process of disintegration of pathogenic organisms giving rise to infection.
Enumeration of methods of sterilization:
There are two methods—physical and chemical.
Physical Methods of sterilization:
- Sunlight
- Drying
- HeatDry heat
- Moist heat
- Ozone
- Radiation
- Filtration.
Chemical Methods of sterilization:
- Phenols
- Oxidizing agents
- Acids and alkalis
- Dyes
- Heavy metals
- Alkylating agents
- Beta-propiolactone
- Surface active agents.
Sterilization by Methods using Moist Heat:
- Moist heat is very efficient in penetrating the material as compared to dry heat.
- Mainly this is used to sterilize culture media before washing, glass syringes, surgical instruments, laboratory coat, aprons, etc.
- The lethal effect of moist heat is by denaturation and coagulation of proteins.
- Moist heat should be given in the following ways, i.e.
- Temperature below 100°C
- Temperature around 100°C
- Temperature above 100°
Temperature below 100°C:
- Pasteurization of milk: Pasteurization is the method which is used for sterilization of the milk. Temperature employed is either 63°C for 30 minutes (holder method) or 72°C for 15 to 20 seconds followed by cooling quickly to 13°C or lower (flash method). Organisms like Mycobacterium, Salmonella, and Brucellae are killed. Only Coxiella burnetii survive the holder’s method which is resistant to it. Spores may not be destroyed.
- Vaccine bath: It is used for killing non-sporing bacteria which may be present in the vaccine. In this method heating is done for 1 hour at 60°C in a vaccine bath.
- Water bath: This is used for sterilization of serum or body fluids which consists of coagulable protein. In this method heating is done for 1 hour at 56°C for several successive days in a water bath.
- Inspissation: This method is used for the sterilization of the Lowenstein–Jensen medium and Loeffl’s serum slope. In this method, heating is done at 80°C for half an hour on three successive days in an inspissator.
- Principle of inspissator:
- On the first day during first exposure, all vegetative forms are killed.
- On the first day spores which do not get killed, germinate to form vegetative forms before the second exposure.
- On the second day as exposure is done newly formed vegetative forms get killed.
- If still spores are present, germinate till the third exposure, and as the third exposure is given on the third day complete sterilization is ensured.
Temperature around 100°C:
- Tyndallization: It is the fractional sterilization method. This is the process by which the medium is placed at 100°C in flowing steam for 30 minutes on three successive days.
The mechanism underlying this method is that vegetative cells get destroyed at 100°C. Tyndallization is applied only to nutrient media.- Principle: One first exposure vegetative forms are killed. Spores that are present germinate on successive days and are killed by the second or third exposure.
- Boiling: Most of the vegetative forms of bacteria are killed at 100°C for 10 to 30 minutes. But this does not ensure complete sterilization as some spore-forming bacteria may not be eliminated completely. In this method addition of 2% sodium carbonate promote sterilization.
This method is recommended for the sterilization of rubber stoppers, cylinders, scissors, pipettes, scalpels, syringes, etc. This method should not be used to sterilize the instruments which are used in the surgical procedures. - Steam at atmospheric pressure: Here free steam is used to sterilize culture media which consists of sugar and gelatin, which may decompose if subjected to the higher temperature. One exposure for 90 minutes provides surety of complete sterilization. Koch or Arnold steam sterilizer is used.
Temperature above 100°C:
- This is the most widely used method for sterilization.
- Sterilization is carried out at a temperature between 108°C to 147°C
- Sterilization is done by generating steam. Autoclave is the most commonly used sterilizer.
Autoclave:
- Principle: Water boils when its vapor pressure equals that of the surrounding atmosphere. Boiling of water occurs at 100°C when its vapor pressure is equal to atmospheric pressure but when pressure inside a close vessel increases the temperature at which water boils also increases. Saturated steam has good penetrating power and microbes are susceptible to moist heat.
When steam comes in contact with a cooler surface it condenses to water and gives latent heat to that surface. Reduction in volume sucks more steam to the area and the process continues till the temperature of that surface is raised to that of steam. Condensed water ensures moist conditions for killing microbes present.
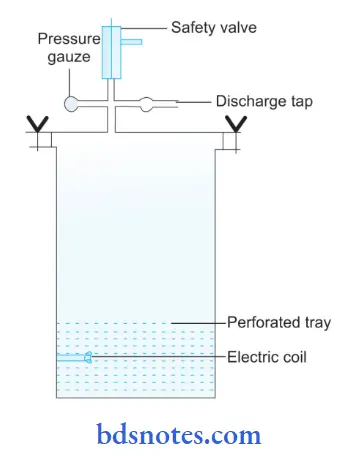
Structure of Autoclave:
- Autoclave has a vertical or horizontal cylinder of stainless steel which is supported by a case made up of an iron sheet.
- The lid or door of an autoclave is heavy and is secured by butterfly nuts, this is rendered airtight by an asbestos washer.
- The lid consists of three screws, i.e. steam outlet, safety valve, and pressure indicator.
- Electricity or gas burner provides heat to the autoclave.
Sterilization Cycles of an Autoclave:

Articles Sterilized by Moist Heat:
Question 3. Write a short note on the hot air oven.
Or
Write the method of sterilization by hot air oven.
Answer:
Hot air oven is the method of sterilization by dry heat.
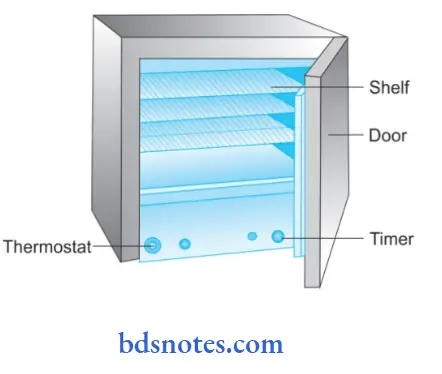
Structure of Hot Air Sterilizer:
- Hot air sterilizer consists of two walled chambers, i.e. outer wall and inner wall.
- The outer wall is made up of asbestos for reducing the radiation of heat.
- The inner wall is of copper vessels.
- Chamber is divided into small compartments by the removable racks.
- The thermometer lies at one of the corners.
- The heating of the oven is done by electricity with heating elements lying inside the wall of the chamber.
Directions for Use:
- The hot air sterilizer should be heated at 160°C for 1 hour or 180°C for half an hour.
- Glassware to be sterilized should be covered by craft paper and should be kept in the chamber in a way that allows free circulation of air between the objects.
- Adjust the temperature at the required level.
- The temperature should be recorded by thermometer and is maintained, i.e. 160°C for 1 hour or 180°C for half an hour.
- As the above-mentioned time gets over, electricity is cut off and the material is allowed to cool.
- After cooling material should be removed from the oven.
Precautions for use of Hot Air Oven:
- It must be fitted with fans to ensure the distribution of hot air.
- It should not be overloaded.The oven must be allowed to cool for about 2 hours before opening the doors, otherwise, glasswares are likely to get cracked.
- The material to be sterilized is dry
- Petri dishes and pipettes are wrapped in paper.
- Rubber material or inflatable material should not be kept.
Uses Of Hot Air Oven:
- For sterilization of glassware, i.e. flask, pipette, test tube, etc.
- For sterilization of scalpel, scissors, forceps, and various other surgical materials.
- For sterilizing the swabs.
- For sterilizing pharmacological products, i.e. liquid paraffin sulphonamides, dusting powder, fat, grease, etc.
Sterilization Control of Hot Air Oven:
- The spores of the non-toxigenic strain of Clostridium tetani are used to test dry heat efficiency.
- Browne’s tube (green spot) is available for sterilization by dry heat. Green color is produced after 60 minutes at 160°C.
- Thermocouples may be used.
Advantages Of Hot Air Oven:
- Economical
- Does not rust metals
- Easily monitor
Disadvantages Of Hot Air Oven:
- Difficult to control the temperature
- Slow penetration
- Rubber and inflatable materials should not be sterilized.
Question 4. Write in brief about the autoclave.
Or
Write a short note on the autoclave.
Or
Write a short note on autoclaving.
Or
Write a short answer on the autoclave.
Answer:
Principle of Autoclave:
Water boils when its vapor pressure equals that of the surrounding atmosphere. Boiling of water occurs at 100°C when its vapor pressure is equal to atmospheric pressure but when pressure inside a close vessel increases the temperature at which water boils also increases. Saturated steam has good penetrating power and microbes are susceptible to moist heat.
When steam comes in contact with a cooler surface it condenses to water and gives latent heat to that surface. Reduction in volume sucks more steam to the area and the process continues till the temperature of that surface is raised to that of steam. Condensed water ensures moist conditions for killing microbes present.
Structure of Autoclave:
- Autoclave has a vertical or horizontal cylinder of stainless steel which is supported by a case made up of an iron sheet.
- The lid or door of an autoclave is heavy and is secured by butterfly nuts, this is rendered airtight by an asbestos washer.
- The lid consists of three screws, i.e. steam outlet, safety valve, and pressure indicator.
- Electricity or gas burner provides heat to the autoclave.
Sterilization Cycles of an Autoclave:
Directions for Use of Autoclave:
- Always place sufficient water in the cylinder.
- Articles that are to be sterilized should be placed on the tray.
- Properly close the lid and tighten the screws.
- Adjust the safety valve to the required pressure.
- Start the heating with the steam tap kept open to displace the air inside
- As all the air inside the chamber is expelled out, the tap should be closed.
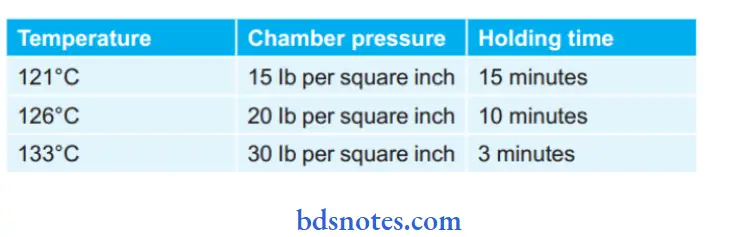
- Record the pressure properly at the proper temperature and timing which is mainly 121°C at 15 Ib per square inch pressure for 15 minutes. This ensures the killing of all microorganisms including spores.
- As the holding period gets over, turn off the heater and the autoclave should be allowed to cool till the pressure is equal to atmospheric pressure.
- Slowly open the steam tap and allow air to enter inside.
- Open the lid and take the material for usage.
Precautions of Autoclave:
- Complete air should be expelled out of the autoclave. The presence of air decreases the temperature of the steam and affects the process of sterilization. Presence of air form air pockets surrounding the material and prevent penetration of steam inside the material.
- Cotton wool plugs should be covered by craft paper or cellophane sheets for avoiding the drenching which is produced by the condensed steam.
- As the inside and outside pressure is equal, the lid should be opened.
When the pressure inside the lid is low then only the lid should be opened.
Uses of Autoclave: Autoclave sterilizes all the materials which should not get damaged by the steam, i.e.
- Surgical instruments
- Swabs
- Syringes and needles
- Saline solution
- Distill water
- Dressing materials
- Laboratory ware and coats
- Pharmaceutical products
- All solid and liquid media
Sterilization Control in an Autoclave:
This is to be done to confirm whether objects or articles are properly sterilized or not. Efficy of the autoclave is determined by:
Biological control of Autoclave:
- In this paper strips are used which are impregnated with.
- Bacillus subtilis or paper strips or ampoules consisting of spores of Bacillus stearothermophilus which are supplied by the material to be sterilized.
- As sterilization is over these strips are transferred to particular media and is incubated.
- The medium should be observed for bacterial growth, if the medium shows growth, sterilization is improper and if it does not show any growth sterilization is perfect.
Physical control of Autoclave:
-
- Various chemical dyes are used which show changes in color at specific temperatures.
- Chemicals of known melting point.
- Melting at a particular temperature indicates whether the temperature had reached the desired level or not
- Use of autoclave tapes and thermocouples.
Advantages of Autoclave:
- Economical
- Good penetration
- Short cycle time
- Easily monitored
- No special chemicals are required for sterilization.
Disadvantages of Autoclave:
- Carbon steel get damaged.
- Presence of moisture retention.
Question 5. Write a short note on pasteurization.
Answer:
- Pasteurization is the method of moist heat sterilization that works at a temperature below 100°C.
- It is the method which is used for the sterilization of the milk.
- It is done by two methods which as follows:
- Holder’s method: In this temperature employed is 63°C for 30 min.
- Flash method: In this temperature employed is 72°C for 15 to 20 seconds followed by quick cooling to 13°C or lower.
- Organisms like Mycobacterium, Salmonella, and Brucellae are killed. Only Coxiella burnetii survive the holder’s method which is resistant to it. Spores may not be destroyed
Question 6. Define and classify methods of sterilization,
Answer:
Sterilization is a process by which an article, surface, or medium is made free of all microorganisms both in vegetative and spore state.
Classification of Methods of Sterilization:
Physical Methods of Sterilization:
- Sunlight
- Drying
- Heat Dry heat
- Moist heat
- Ozone
- Radiation
- Filtration
Chemical Methods of Sterilization:
- Phenols
- Oxidizing agents
- Acids and alkalis
- Dyes
- Heavy metals
- Alkylating agents
- Beta-propiolactone
- Surface active agents
Sterilization definition:
Question 7: Describe various methods of sterilization by heat.
Answer:
Methods of sterilization by heat is of two types, i.e. dry heat sterilization and moist heat sterilization
1. Dry Heat Sterilization
- Dry heat sterilization should be preferred for the sterilization of glass syringes, test tubes, Petri dishes, and materials such as oils, jelly, and powder.
- As compared to moist heat sterilization, dry heat sterilization is less efficient and needs a long time and high temperature for sterilization.
- The penetration power of dry heat is low, so it is not effective when non-conducting materials protect microorganisms.
Killing by dry heat is due to:
- Protein denaturation
- Oxidative damage
- Toxic effect of elevated levels of electrolytes.
Following are the procedures by which dry heat can be used:
- Red heat: In this method direct heating of an instrument or object in a flame is done till it becomes red hot. The method has limited applications. It is used for sterilizing non – inflammable materials. For example, Inoculating wires or loops, needles, forceps, scissors, spatula, etc.
- Flaming: The article is passed over a flame without allowing it to become red hot. Its efficacy is not certain. It is used to sterilize the mouth of cultured tubes, cotton wool plugs, and glass slides.
- Incineration: In this method, direct burning of material should be carried out at a high temperature, i.e. 800 to 1,000°C. This is an excellent method for sterilization and rapidly destroying contaminated materials by direct burning. It is used for destroying materials, for example, Soiled dressing, Bedding, Pathological materials, etc.
- Hot air oven: This method is the most common and widely used method of sterilization by dry heat.
Structure of Hot Air Oven:
- Hot air sterilizer consists of two walled chambers, i.e. outer wall and inner wall.
- The outer wall is made up of asbestos for reducing the radiation of heat.
- The inner wall is of copper vessels.
- Chamber is divided into small compartments by the removable racks.
- The thermometer lies at one of the corners.
- The heating of the oven is done by electricity with heating elements lying inside the wall of the chamber.
Directions for Use of Hot Air Oven:
- The hot air sterilizer should be heated at 160°C for 1 hour o 180°C for half an hour.
- Glassware to be sterilized should be covered by craft paper and should be kept in the chamber in a way that allows free circulation of air between the objects.
- Adjust the temperature at the required level.
- The temperature should be recorded by thermometer and is maintained, i.e. 160°C for 1 hour or 180°C for half an hour.
- As the above-mentioned time get over, electricity is cut of and the material is allowed to cool.
- After cooling material should be removed from the oven.
Precautions for Use of Hot Air Oven:
- It must be fitted with fans to ensure the distribution of hot air.
- It should not be overloaded.
- The oven must be allowed to cool for about 2 hours before opening the doors, otherwise, glasswares are likely to get cracked.
- The material to be sterilized is dry.
- Petri dishes and pipettes are wrapped in paper.
- Rubber material or inflatable material should not be kept.
Uses Of Hot Air Oven:
- For sterilization of glassware, i.e. flask, pipette, test tube, etc.
- For sterilization of scalpel, scissors, forceps, and various other surgical materials.
- For sterilizing the swabs
- For sterilizing pharmacological products, i.e. liquid paraffin sulphonamides, dusting powder, fat, grease, etc.
2. Moist Heat Sterilization
Sterilization by methods using moist heat.
- Moist heat is very efficient in penetrating the material as compared to dry heat.
- Mainly this is used to sterilize culture media before washing, glass syringes, surgical instruments, laboratory coat, aprons, etc.
- The lethal effect of moist heat is by denaturation and coagulation of proteins.
- Moist heat should be given in the following ways, i.e.
- Temperature below 100°C
- Temperature around 100°C
- Temperature above 100°C
Temperature below 100°C:
- Pasteurization of milk: Pasteurization is the method which is used for sterilization of the milk. The temperature employed is either 63°C for 30 minutes (holder method) or 72°C for 15 to 20 seconds followed by cooling quickly to 13°C or lower (flash method). Organisms like Mycobacterium, Salmonella, and Brucellae are killed. Only Coxiella burnetii survive the holder’s method which is resistant to it. Spores may not be destroyed.
- Vaccine bath: It is used for killing non-sporing bacteria which may be present in the vaccine. In this method heating is done for 1 hour at 60°C in a vaccine bath.
- Water bath: This is used for the sterilization of serum or body fluids which consists of coagulable protein. In this method heating is done for 1 hour at 56°C for several successive days in a water bath.
- Inspissation: This method is used for the sterilization of the Lowstein-Jensen medium and Loeffl’s serum slope. In this method, heating is done at 80°C for half an hour on three successive days in an inspissator.
- Principle of Inspissator:
- On the first day during first exposure, all vegetative forms are killed.
- On the first day spores which do not get killed, germinate to form vegetative forms before the second exposure.
- On the second day as exposure is done newly formed vegetative forms get killed.
- If still spores are present, germinate till the third exposure, and as the third exposure is given on the third day complete sterilization is ensured.
Temperature around 100°C:
- Tyndallization: It is the fractional sterilization method. This is the process by which the medium is placed at 100°C in flowing steam for 30 minutes on three successive days. The mechanism underlying this method is that vegetative cells get destroyed at 100°C. Tyndallization is applied only to nutrient media.
- Principle: One first exposure vegetative forms are killed. Spores that are present germinate on successive days and are killed by the second or third exposure.
- Boiling: Most of the vegetative forms of bacteria are killed at 100°C for 10 to 30 minutes. But this does not ensure complete sterilization as some spore-forming bacteria may not be eliminated completely.
- In this method addition of 2% sodium carbonate promote sterilization.
- This method is recommended for the sterilization of rubber stoppers, cylinders, scissors, pipettes, scalpels, syringes, etc.
- This method should not be used to sterilize the instruments which are used in the surgical procedures.
- Steam at atmospheric pressure: Here free steam is used to sterilize culture media which consists of sugar and gelatin, which may decompose if subjected to a higher temperature. One exposure for 90 minutes provides surety of complete sterilization. Koch or Arnold steam sterilizer is used.
Temperature above 100°C:
- This is the most widely used method for sterilization.
- Sterilization is carried out at a temperature between 108°C to 147°C
- Sterilization is done by generating steam. Autoclave is the most commonly used sterilizer.
Autoclave:
Principle of Autoclave:
Water boils when its vapor pressure equals that of the surrounding atmosphere. Boiling of water occurs at 100°C when its vapor pressure is equal to atmospheric pressure but when pressure inside a close vessel increases the temperature at which water boils also increases.
Saturated steam has good penetrating power and microbes are susceptible to moist heat. When steam comes in contact with a cooler surface it condenses to water and gives latent heat to that surface.
Reduction in volume sucks more steam to the area and the process continues till the temperature of that surface is raised to that of steam. Condensed water ensures moist conditions for killing microbes present.
Structure of autoclave:
- Autoclave has a vertical or horizontal cylinder of stainless steel which is supported by a case made up of an iron sheet.
- The lid or door of an autoclave is heavy and is secured by butterfly nuts, this is rendered airtight by an asbestos washer.
- The lid consists of three screws, i.e. steam outlet, safety valve, and pressure indicator.
- Electricity or gas burner provides heat to the autoclave.
Sterilization cycles of an autoclave:

Question 8. Write a short note on the functions and uses of the autoclave.
Answer:
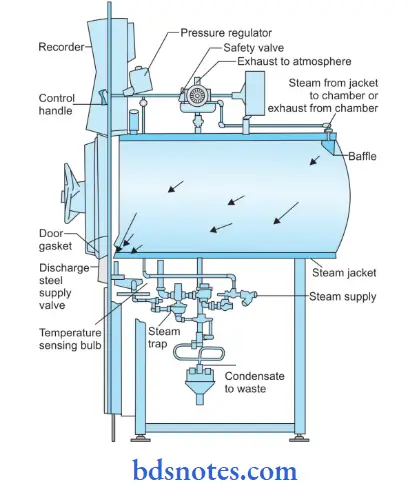
Function of Autoclave:
- In its simplest form, the laboratory autoclave consists of a vertical or horizontal cylinder of gun metal or stainless steel, in a supporting sheet iron case.
- The lid or door is fastened by screw clamps and made airtight by an asbestos washer.
- The autoclave has on its lid or upper side= a discharge tap for air and steam, a pressure gauge, and a safety valve that can be set to blow off at any desired pressure.
- Heating is by gas or electricity.
- Sufficient water is put in the cylinder, the material to be sterilized is placed on the tray and the autoclave is heated.
- The lid is screwed tight with the discharge tap open.
- The safety valve is adjusted to the required pressure.
- The steam-air mixture is allowed to escape freely till all the air has been displaced. This can be tested by leading the escaping steam into a bucket of water through rubber tubing
- When no more air bubbles come out of the bucket the discharge tap is closed.
- The steam pressure rises inside and when it reaches the desired set level, the safety valves open, and the excess steam escapes.
- From this point, the holding period is calculated.
- When the holding period is over, the heater is turned off and the autoclave is allowed to cool till the pressure gauge indicates that the pressure inside is equal to the atmospheric pressure.
- The discharge tap is opened slowly and the air is let into the autoclave.
- The lid is now opened and the sterilized material is removed.
Uses of Autoclave:
Autoclave sterilizes all the materials which should not get damaged by the steam, i.e.
- Surgical instruments
- Swabs
- Syringes and needles
- Saline solution
- Distill water
- Dressing materials
- Laboratory ware and coats
- Pharmaceutical products All solid and liquid
Question 9. Define sterilization. Describe methods of sterilization and disinfection in the dental clinic.
Answer:
Sterilization definition: Sterilization is a process by which an article, surface, or medium is made free of all microorganisms both in vegetative and spore states.
Sterilization Methods
Sterilization is a process by which an article, surface, or medium is made free of all microorganisms both in vegetative and spore states. A number of sterilization methods are available for heat-tolerant dental instruments. These include use of steam under pressure (steam autoclave), dry heat, or unsaturated chemical vapor.
The duration of sterilization, temperatures, and other operating parameters recommended by the equipment manufacturer should be used. Additionally, instructions for use of correct containers, wraps, and chemical or biological indicators should always be followed.
Heat sterilization methods, i.e. steam autoclave, dry heat, and unsaturated chemical vapor are preferred for all equipment that can withstand high temperatures for several reasons:
- Effective
- Relatively easy to use
- Comparatively inexpensive
- Readily monitored for effectiveness.
Liquid chemical disinfectants/sterilants should be used only when heat will damage an item.
1. Steam Sterilization:
Among sterilization methods, steam sterilization is the most widely used for wrapped and unwrapped critical and semicritical items that are not sensitive to heat and moisture.
When using an autoclave, the load must be placed so that steam can circulate freely around each item, because steam must be able to reach all instrument surfaces at a required temperature and pressure for a specified time in order to kill all microorganisms and achieve sterilization. Be sure to follow the autoclave manufacturer’s operating instructions.
Autoclave of Steam Sterilization:
- An autoclave is a self-locking machine that sterilizes with steam under pressure, achieved by high temperature.
- Autoclaves are the universally accepted means of sterilization.
- It is generally accepted that an autoclave chamber must reach at least 121°C at 15 P si for a minimum of 30 minutes to ensure adequate sterilization.
- Sterilization time may vary depending on the quantity and density of items in the autoclave chamber. Overloading must be avoided.
- Instruments and materials for sterilizing in the autoclave are usually enclosed in muslin wrappers as surgical packs.
- These packs should be porous to allow steam to penetrate and reach the instruments.
- The autoclave is employed for the sterilization of instruments, extraction forceps, surgical instruments, explorers, etc. The sterilized instruments should remain wrapped until next used.
Advantages of Steam Sterilization:
- Is quick and easy to use.
- Allows loads to be packaged, making it easier to maintain items in a sterile state.
- Penetrates fabric and paper wrappings.
- Can be readily monitored for effectiveness.
- Is economical and very reliable.
Disadvantages of Steam Heat Sterilization:
- May cause rust and corrosion (corrosion inhibitors such as sodium nitrite are available that may reduce this problem).
- May damage plastics.
- May blunt certain sharp items.
2. Dry Heat Sterilization:
Dry heat is used to sterilize materials that might be damaged by moist heat (for example dental burs and certain orthodontic instruments). Although dry heat has the advantages of low operating costs and being non-corrosive, it is a prolonged process and the high temperatures required are not suitable for certain patient care items and devices with temperatures ranging from 300° F (149°C) and upward can be used for sterilization.
Dry heat sterilizers used in dentistry include static-air and forced-air types. The static-air type is commonly called an oven type sterilizer. The forced-air type is also known as a rapid heat transfer sterilizer. Heated air is circulated through the chamber at a high velocity, permitting more rapid transfer of energy from the air to the instruments, thereby reducing the time needed for sterilization compared to the oven-type sterilizer.
Advantages of Dry Heat Sterilization:
- Is very reliable
- Rust and corrosion are not a problem, provided that items are dry prior to sterilization.
- Is easy to use and requires little maintenance.
- Can be readily monitored for effectiveness.
Disadvantages of Dry Heat Sterilization:
- Usually requires longer processing times than steam sterilization or unsaturated chemical vapor.
- Damages some plastics
- Requires careful loading
- High temperatures may prohibit the use of some materials and may melt or destroy some metal or solder joints.
3. Unsaturated Chemical Vapor Sterilization:
Unsaturated chemical vapor sterilization involves heating a chemical solution of primary alcohol with formaldehyde in a pressurized chamber. This method of sterilization is ideally suited to carbon steel instruments (for example, Dental burs) because the low level of water present during the cycle results in less corrosion than might be expected with steam sterilization. Instruments must be dry before sterilization.
Follow the manufacturer’s instructions.
Advantages of Chemical Vapor Sterilization:
- Is relatively quick.
- Does not rust or corrode metal items. Is very reliable.
- Can be used with packaged items (paper packaging only).
- Can be monitored for effectiveness.
Disadvantages of Chemical Vapor Sterilization:
- Requires good ventilation owing to fumes. Would not penetrate fabric-wrapped packs. Damages some plastics.
- Requires replacement of special solution, increasing cost.
- Requires hazardous waste disposal of the sterilizing solution.
4. Sterilization of Unwrapped Instruments:
An unwrapped sterilization cycle (sometimes called flash sterilization) is a method of sterilizing patient care items for immediate use. Critical and semi-critical items that have been sterilized unwrapped should be transferred immediately using an aseptic technique for the ultimate use. The unwrapped sterilization cycle in tabletop sterilizers is usually preprogrammed by the manufacturer to a specific time and temperature setting. Thorough cleaning and drying of instruments precede the unwrapped cycle.
Mechanical monitors are checked and chemical indicators are used for each cycle. Care is taken to avoid thermal injury to dental workers and patients. Items are handled and transported aseptically to the point of use to maintain sterility. As implantable devices should be quarantined after sterilization until the results of biological monitoring are known, unwrapped or flash sterilization of implantable devices is not recommended.
Bead sterilizers: Historically, bead sterilizers have been used in dentistry to sterilize small metallic instruments (for example. Endodontic fies). This method employs a heat transfer device. The media used are glass beads or salt and the temperature achieved is 220°C. The method employs the submersion of small instruments such as endodontic files and burs into the beads; they are sterilized in 10 seconds provided they are clean.
Methods of Disinfection
Liquid chemical germicides: Heat-sensitive critical and semi-critical instruments and devices can be sterilized by immersing them in liquid chemical germicides. However, items sterilized in this manner can require approximately 12 hours of complete immersion. Additionally, items sterilized in this manner must be rinsed with sterile water to remove any toxic or irritating residues, handled using sterile gloves and dried with sterile towels, delivered to the point of use in an aseptic manner, and then used immediately.
Because of these limitations, they are almost never used to sterilize instruments. Rather, these chemicals are more often used for high-level disinfection of heat-sensitive semi-critical instruments and devices. Shorter immersion times (12–90 minutes) make high-level disinfection more practical than sterilization; however, instruments and devices disinfected in this manner must still be handled as if sterile (for example rinsed with sterile water, dried with sterile towels, etc.) and used immediately.
Chemical sterilants (for example Glutaraldehyde, peracetic acid, hydrogen peroxide) are powerful, sporicidal chemicals and are highly toxic. Instruments can be sterilized by placing them in a 2% solution of glutaraldehyde for 6-10 hours. The chemical sterilants must only be used according to the manufacturer’s instructions and for those applications indicated on their label. Misapplications include the use of an environmental surface disinfectant or instrument-holding solution.
Alcohols are effective as skin antiseptics. Usually, a 50% to 80% ethyl alcohol solution is recommended. In general, the use of heat-sensitive semi-critical items that must be processed with liquid chemical germicides is discouraged; heat-tolerant or disposable alternatives are available for the
majority of such items.
Surface Infection Control
In the dental office, surface contamination can occur during patient care. Certain surfaces especially the ones touched regularly during patient care (for example, Light handles, Unit switches, and Drawer knobs can serve as reservoirs of microbial contamination). These surfaces can be divided into clinical contact surfaces and housekeeping surfaces.
Clinical Contact Surface
Clinical contact surfaces can be directly contaminated from patient materials either by direct spray or spatter generated during dental procedures or by contact with dental professionals’ gloved hands.
These surfaces can subsequently contaminate other instruments, devices, hands, or gloves.
- Examples of such surfaces include:
- Light handles
- Switches
- Dental radiograph equipment
- Dental chairside computers
- Reusable containers of dental materials
- Drawer handles
- Faucet handles
- Countertops
- Pens
- Telephones
- Doorknobs.
- Barrier protection of surfaces and equipment can prevent contamination of surfaces that are difficult to clean. Barriers include using clear plastic wrap, bags, sheets, tubing, and plastic-backed paper or other materials impervious to moisture.
- These coverings can become contaminated. They should be removed and discarded between patients, while dental health care professionals (DHCP) are still gloved. After removing the barrier, examine the surface to make sure it did not become soiled.
The surface needs to be cleaned and disinfected only if contamination is evident. Otherwise, after removing gloves and performing hand hygiene, DHCP should place clean barriers on these surfaces before the next patient. - If barriers are not used, surfaces should be cleaned and disinfected between patients by using a disinfectant with an HIV, HBV claim (i.e. low-level disinfectant) or a tuberculocidal claim (i.e. intermediate-level disinfectant). Intermediate-level disinfectant should be used when the surface is visibly contaminated with blood or other fluids.
- General cleaning and disinfection are recommended for surfaces, dental unit surfaces, and countertops at the end of daily activities and these may have become contaminated since their last cleaning. To facilitate daily cleaning, treatment areas should be kept free of unnecessary equipment and supplies.
- Manufacturers of dental devices and equipment should provide information regarding material compatibility with liquid chemical germicides, whether the equipment can be safely immersed for cleaning, and how it should be decontaminated if servicing is required.
- Because of the risks associated with exposure to chemical disinfectants and contaminated surfaces, dental professionals who perform environmental cleaning and disinfection should wear gloves and other PPE to prevent occupational exposure to infectious agents and hazardous chemicals.
- Chemical and puncture-resistant utility gloves off more protection than patient examination gloves when using hazardous chemicals.
Housekeeping Surfaces
Evidence does not support that housekeeping surfaces (for example, Flors, Walls, and Sinks) pose a risk for disease transmission in dental healthcare settings.
- These need to be cleaned only with a detergent and water or a disinfectant/detergent depending on the nature of the surface, type, and degree of contamination.
- Schedules and methods vary according to the area (for example, Dental operatory, laboratory, bathrooms, or reception rooms), surface, amount, and type of contamination.
- Floors should be cleaned regularly and spills should be cleaned up promptly. A disinfectant/detergent designed for general housekeeping purposes should be used in patient care areas if uncertainty exists regarding the nature of the soil on the surface (for example Blood or body fluid contamination versus routine dust or dirt).
- However, when housekeeping surfaces are visibly contaminated by blood or OPIUM, prompt removal and surface disinfection is essential. Part of the cleaning strategy is to minimize contamination of cleaning solutions and cleaning tools (for example, Mop heads or Cleaning cloths).
- Mops and cloths should be cleaned after use and allowed to dry before reuse or single-use, disposable mop heads, and cloths should be used to avoid spreading contamination.
Handpieces and Other Devices
Multiple semi-critical dental devices that touch mucous membranes are attached to the air or waterlines of the dental unit. Among these devices are high- and low-speed handpieces, prophylaxis angles, ultrasonic and sonic scaling tips, air abrasion devices, and air and water syringe tips.
These devices have the potential for retracting oral fluids into their internal compartments. Restricted physical access limits their cleaning. This indicates that retained patient material can be expelled intraorally during subsequent uses.
- Any dental device connected to the dental air/water system that enters the patient’s mouth should be run to discharge water, air or a combination for a minimum of 20-30 seconds after each patient.
- This procedure physically flushes outpatient material that might have entered the turbine, air, and waterlines. Heat methods can sterilize dental handpieces and other intraoral devices attached to air or waterlines.
- Proper scrubbing with detergent, water, and drying followed by wiping with a suitable chemical disinfectant is essential for those ultrasonic scalers, handpieces, and air syringes that cannot be sterilized.
- For processing any dental device that can be removed from the dental unit air or waterlines, neither surface disinfection nor immersion in chemical germicides is an acceptable method.
- Manufacturers’ instructions for cleaning, lubrication, and sterilization should be followed to ensure both the effectiveness of the process and the longevity of handpieces.
- Some components of dental instruments are permanently attached to dental unit waterlines and although they do not enter the patient’s oral cavity, they are likely to become contaminated with oral flids during treatment procedures.
- Components (for example Handles or dental unit attachments of saliva ejectors, high-speed air evacuators, and air/water syringes) should be covered with impervious barriers that can be changed after each use.
- If the item becomes visibly contaminated during use, the dental practitioner should clean and disinfect it with a disinfectant (intermediate-level) before use on the next patient.
Saliva Ejectors:
- Backflow from low-volume saliva ejectors occurs when the pressure in the patient’s mouth is less than that in the evacuator.
- Backflow in low-volume suction lines can occur and microorganisms present in the lines retracted into the patient’s mouth when a seal around the saliva ejector is created (for example By a patient closing lips around the tip of the ejector, creating a partial vacuum). This backflow can be a potential source of cross-contamination.
- Although no adverse health effects associated with the saliva ejector have been reported, practitioners should be aware that in certain situations, backflow can occur when using a saliva ejector
Question 10. Differentiate between sterilization, disinfection, and antisepsis with examples (if any).
Answer:


Leave a Reply