Regulation Of Gene Action In Bacteria And Viruses
Bacteria are exposed to a wide range of environmental conditions. For example, E. coli may encounter rapidly changing growth conditions as they pass from the mammalian intestinal tract to sewer systems to polluted rivers, lakes, ponds, and so on.
- Each of these ecological niches will provide different organic molecules for use as energy sources. From this fact, it can be concluded that natural selection will have preserved those organisms that have evolved ways of adapting; to the wide range of environmental conditions encountered f during their evolution.
- Indeed, the available information f indicates that most prokaryotes such as E. coli exhibit remarkable capacities to adapt to diverse environmental; conditions.
- To a great extent, the adaptability of bacteria and other? prokaryotes depend on their ability to “turn on” and “turn off’ the expression of specific sets of genes in response to the specific demands of the environmental milieu (i.e., surroundings).
- In other words, prokaryotes exhibit an outstanding ability to regulate the expression of specific genes in response to environmental signals.
The expression of particular genes is “turned on” when the products of these genes are needed for growth in a given environment. Their expression is “turned old when their products are no longer needed for growth in the existing environmental surroundings.
- The ability of an organism to regulate gene expression in this way will increase its overall “fitness’ (i.e., its ability to grow and leave progeny under a variety of environmental conditions).
- The synthesis of gene transcripts (i.e., various types of RNA molecules) and translation products (i.e., proteins) require the expenditure of a great amount of energy.
- The chromosome of the bacterium Escherichia coli, a single-celled organism, consists of a single circular DNA molecule of about 4.6 × 106 nucleotide pairs. This DNA encodes approximately 4300 proteins, although only a fraction of these are made at any one time.
- The expression of many of them is regulated according to the available food in the environment (see Alberts et al, 2002).
By “turning off the expression of genes when their products are not needed, an organism can avoid wasting energy to synthesize products that maximize the growth rate in the existing environmental surroundings.
Here, one can ask three sorts of questions:
- What are the mechanisms by which these organisms regulate gene expression in response to changes in the environment?
- Is there a single mechanism by which the expression of different genes or sets of genes are regulated?
- Or are different genes controlled by different mechanisms?
Some genes, for example, the genes specifying ribosomal RNAs, ribosomal proteins and transfer RNAs, are certainly expressed at some time in virtually all cells regardless of the environmental conditions. The products of these genes are required for the growth of all cells in all environments.
- However, the products of many other genes are required for growth only in certain environments, and the expression of these genes is regulated such that the products are synthesized only when they are needed.
- As a result, the expression of these genes is continually being “turned on” and “turned off’ in response to changes in the environment.
- From this discussion, one can conclude that gene expression can be (and is) regulated at several different levels: for example, transcription, mRNA processing, mRNA turnover, translation, and enzyme function.
- However, extensive data indicate that the regulation of transcription is the most important mode of control of gene expression, at least in prokaryotes.
- The regulatory mechanisms of transcription in both prokaryotes and eukaryotes are of two basic types. The first, and best-understood, category includes mechanisms involved in the rapid turn-on and turn-off of gene expression in response to environmental changes.
- Such sort of mechanisms are very significant in microorganisms (bacteria) because these organisms are exposed to sudden changes in the environment.
They provide microorganisms with a great deal of plasticity, and an ability to rapidly adjust their metabolic processes to achieve maximal growth and reproduction under highly variable environmental conditions.
- These quick-responding on-off switches seem to be less important in higher eukaryotes. This might be expected since the circulatory systems of higher eukaryotes buffer their cells against many sudden environmental changes.
- The second major category of regulatory mechanisms includes the so-called preprogrammed circuits of gene expression. In these cases, some event (for example., infection by a virus) triggers the expression of one set of genes.
- The product (or products) of one (or more) of these genes function by turning off the transcription of the first set of genes and/or turning on the transcription of the second set of genes.
- In turn, one or more of the products of the second set act by turning on a third set, and so on. In these cases, the sequential expression of genes is genetically preprogrammed, and the genes usually cannot be turned on out of sequence. Such preprogrammed sequences of gene expression in viral infections are well worked out.
- In most of these preprogrammed sequences, it seems the circuit is cyclical. For example, in viral infections, some event associated with the packaging of the viral DNA or RNA inside the protein coat somehow seems to reset the program so that the first set of genes will again be expressed when a progeny virus subsequently infects another host cell.
Discovery Of Prokaryotic Gene Regulatory Proteins: Genetic analyses in bacteria carried out in the 1950s provided the first evidence for the existence of gene regulatory proteins that turn specific sets of genes on or off. One of these regulators, the lambda repressor, is encoded by a bacterial virus, the bacteriophage lambda.
- The repressor shuts off the viral genes that code for the protein components of new virus particles and thereby enables the viral genome to remain a silent passenger in the bacterial chromosome, multiplying with the bacterium when conditions are favorable for bacterial growth. The lambda repressor was among Gene regulatory proteins.
- the first gene regulatory proteins to be characterized. Other bacterial regulators respond to nutritional conditions by shutting off genes encoding specific sets of metabolic enzymes when they are not needed.
- For example, lac repressor, the first of these bacterial proteins to be recognized, turns off the production of the proteins responsible for lactose metabolism when this sugar is absent from the medium.
- The first step toward understanding gene regulation was the isolation of mutant strains of bacteria and bacteriophage lambda that were unable to shut off specific sets of genes.
- It was proposed at the time, and later proven, that most of these mutants were deficient in proteins acting as specific repressors for these sets of genes.
- Because, these proteins, like most gene regulator proteins, are present in small quantities, it was difficult and time-consuming to isolate them. They were eventually purified by fractionating cell extracts.
- Once isolated, the proteins were shown to bind to specific DNA sequences close to the genes that they regulate.
- The precise DNA sequences that they recognized were then determined by a combination of classical genetics, DNA sequencing, and DNA footprinting experiments.
Constitutive Genes And Inducible Genes
Certain gene products, such as tRNA molecules, rRNA molecules, ribosomal proteins, RNA polymerase components (polypeptides), and other enzymes catalyzing metabolic processes that are commonly known for cellular “housekeeping” functions, are essential components of almost all living cells.
- Genes that specify products of this type are continually being expressed in most cells. Such genes are said to be expressed constitutively and are called constitutive genes.
- The rest of the gene products are needed for cell growth only under certain environmental conditions. Constitutive synthesis of such gene products would be wasteful, using energy that could otherwise be utilized for more rapid growth and reproduction under the existing environmental conditions.
- The evolution of regulatory mechanisms that would provide for the synthesis of such gene products only when and where they were needed would provide organisms with a selective advantage over organisms lacking these mechanisms.
- This undoubtedly explains why presently existing organisms, including the “primitive” bacteria and viruses, exhibit highly developed and very efficient mechanisms for the control of gene expression.
- E.coli and most other bacteria are capable of growth using any one of several carbohydrates (for example., glucose, sucrose, galactose, arabinose, lactose) as an energy source. If glucose is present in the environment, it will be preferentially metabolized by E. coli cells. In the absence of glucose, however, E. coli cells can grow very well on other carbohydrates.
- Cells of E. coli growing in a medium containing the sugar lactose, for example, as the sole carbon source synthesize two enzymes, β-galactosidase and β-gaIactoside permease, that they are uniquely required for the catabolism of lactose (Note: A third enzyme, α-galactoside transacetylase is also synthesized).
- The enzyme β- galactosidase cleaves lactose into glucose and galactose, and the enzyme β-galactoside permease pumps α-galactosides into the cell.
- Neither of these enzymes is of any use to E. coli cells when present in an environment not containing lactose. The synthesis of these two enzymes, of course, requires the utilization of a great amount of energy (in the form of ATP and GTP).
- Thus, E. coli cells have evolved a regulatory mechanism by which the synthesis of these lactose catabolic enzymes is turned on in the presence of lactose and turned off in its absence. In natural environments (such as intestinal tracts and sewers), E. coli cells probably encounter an absence of glucose and the presence of lactose relatively rarely.
- Most of the time, therefore, E. coli cells growing on a carbohydrate other than lactose are transferred to a medium containing lactose as the only carbon source, they rapidly begin synthesizing the enzymes required for lactose utilization.
- This process, by which the expression of genes is turned on in response to a substance in the environment, is called induction. Genes whose expression is so regulated are called inducible genes.
- The substances or molecules responsible for induction are known as inducers (for example., in this case, lactose is inducer).
- Enzymes that are involved in catabolic (i.e., degradative) pathways, such as in lactose, galactose, or arabinose utilization, are characteristically inducible. Research made it evident that induction occurs at the level of transcription.
- Induction alters the rate of synthesis of enzymes, not the activity of existing enzyme molecules. Induction should not be confused with enzyme activation, in which the binding of a small molecule to an enzyme increases the activity of the enzyme (but does not affect its rate of synthesis).
- Further, E. coli and other bacteria possess the metabolic capacity to synthesize most of the organic molecules (such as amino acids, purines, and vitamins) required for their growth.
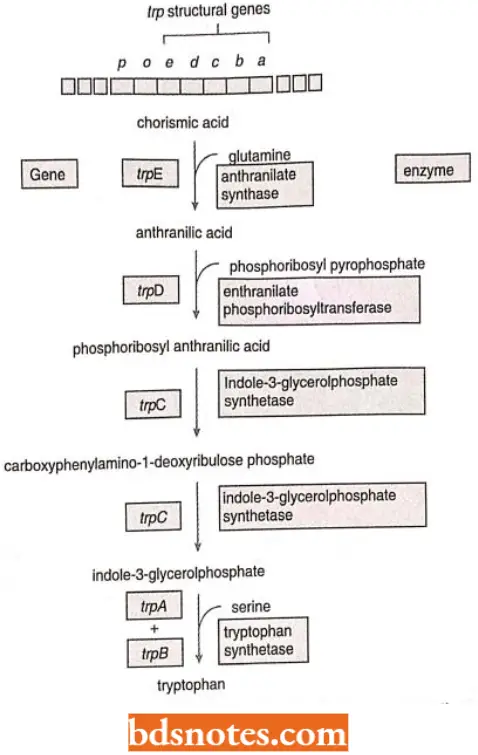
- For example, E. coli has five genes coding for enzymes that are required in the synthesis of tryptophan (an amino acid).
These five genes must be expressed in E. coli cells growing in an environment devoid of tryptophan to provide adequate amounts of this amino acid for ongoing protein synthesis.
- When E. coli cells are present in an environment containing concentrations of tryptophan sufficient to support optimal growth, the continued synthesis of the tryptophan biosynthetic enzymes would be a waste of energy, because these bacteria can take in external tryptophan.
- Thus, a regulatory mechanism has evolved in E. coli by which the synthesis of the tryptophan is present in the external milieu (surrounding environment).
- This process of “turning off’ the expression of sets of genes is called repression. A gene whose expression has been turned off in this way is said to be repressed; when its expression is turned on, a gene of this type is said to be depressed.
- Enzymes that are components of anabolic (biosynthetic) pathways are frequently subject to repression (are repressible).
- Repression, like induction, occurs at the level of transcription. However, repression should not be confused with feedback inhibition, in which the binding of an end product to the first enzyme in a biosynthetic pathway inhibits the activity of the enzyme (but does not affect its synthesis).
Transcriptional Control Mechanisms
In bacteria, there occur several mechanisms of gene regulation at the level of transcription. A notable method depends on whether the enzymes being regulated act in catabolic (degradative) or anabolic (synthetic) metabolic pathways.
- For example, in a multistep catabolic system, the availability of the molecule to be degraded commonly determines whether the enzymes in the pathway will be synthesized. In contrast, in a biosynthetic pathway, the final product is often the regulatory molecule.
- Even when a single protein molecule is translated from a monocistronic mRNA molecule, the protein may be autoregulated, i.e., the protein itself may inhibit initiation of transcription and high concentrations of the protein may cause less transcription of the mRNA that encodes the protein.
- The molecular mechanisms for each of the regulatory patterns differ greatly and are of the following two types – negative regulation and positive regulation. In a negatively regulated system, an inhibitor is present in the cell and prevents transcription.
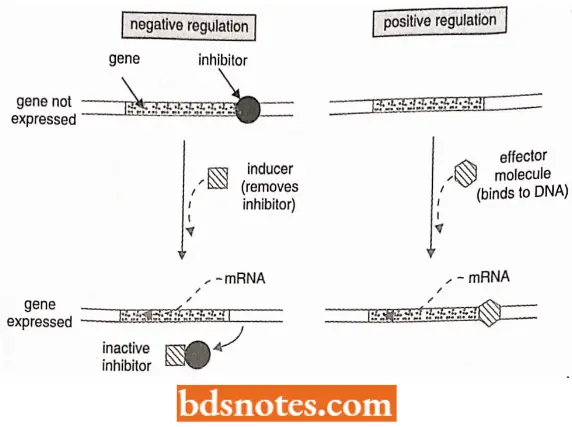
- An antagonist of the inhibitor, called an inducer, is needed to allow initiation of transcription. In a positively regulated system, an effector molecule (which may be a protein, a small molecule, or a molecular complex) activates a promoter; no inhibitor must be abolished.
- Negative and positive regulation are not mutually exclusive, and some systems are both positively and negatively regulated, utilizing two regulators to respond to different conditions in the cell. Thus, a catabolic system may be regulated positively or negatively.
- In a biosynthetic (anabolic) pathway, the final product usually regulates negatively its synthesis; in the simplest type of negative regulation, the absence of the product increases its synthesis, and the presence of the product decreases its synthesis.
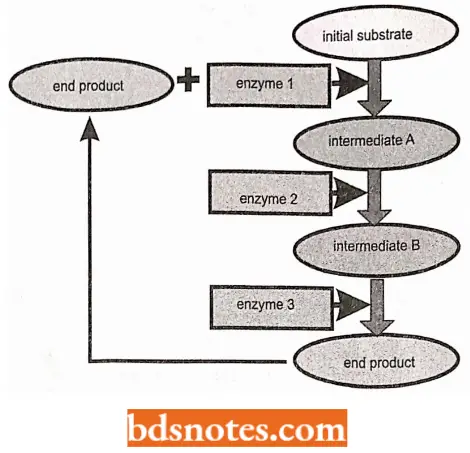
The Operon Model: Induction and repression of gene expression can be accomplished by essentially the same mechanism.
- This mechanism was first accurately described in 1961 when Francois Jacob and Jacques Monod, both 1965 Nobel Prize recipients, proposed the operon model to explain the regulation of genes encoding the enzymes required for lactose utilization in E. coli.
- Jacob and Monod proposed that the transcription of one or a set of contiguous structural genes (i.e. genes coding for polypeptides) is regulated by two controlling elements.
- One of these controlling elements called the regulator gene (or repressor gene), codes for a protein called the repressor; under the appropriate conditions, the repressor binds to the second element, the operator.
- The operator is always located contiguous to the structural gene or genes whose expression it regulates.
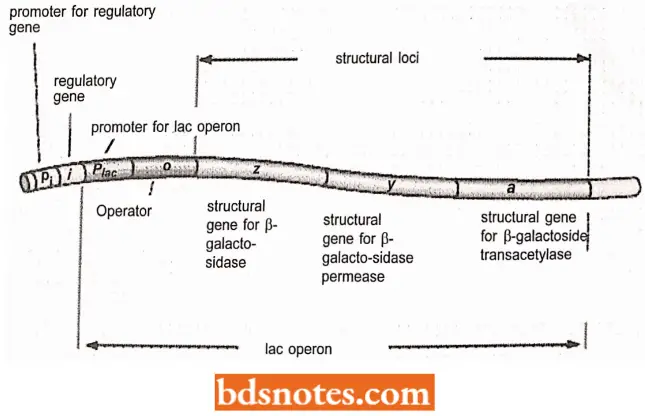
- When the repressor is bound to the operator, transcription of the structural genes cannot occur, We now know that this results because the binding of the repressor to the operator stoanettlly prevents RNA polymerase (enzyme) from binding at the promoter site (the RNA polymerase binding site), which is always located contiguous with the operator sequence.
- The operator is usually located between the promoter and structural genes. (Note: The promoter was not recognized at the time of Jacob and Monod’s proposal but has since been shown to be an essential component of an operon).
- Thus, the complete contiguous unit, including the structural genes, the operator, and the promoter is called the operon (Gardner et al, 2002).
- In other words, an operon is a unit of prokaryotic gene expression that includes co-ordinately regulated (structural) genes and control elements (promoter operator) which are recognized by regulatory gene products (furnace et al., 2000).
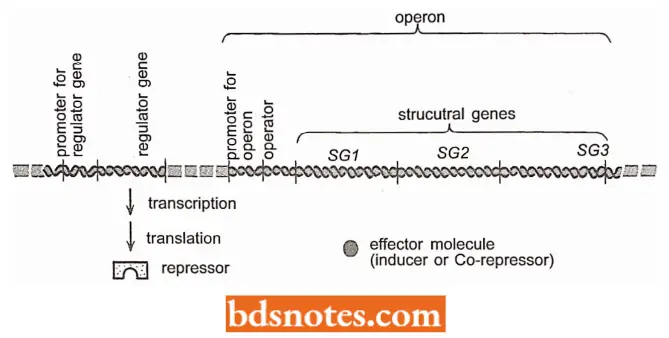
- Whether the repressor will bind to the operator and turn off the transcription of the structural genes in an operon is determined by the presence or absence of effector molecules (small molecules such as amino acids and sugars) in the environment.
- In the case of inducible operons (for example., lac operon), these effector molecules are called inducers. Those active on repressible operons (for example., trp operon) are called co-repressors and act by binding to (or forming a complex with) the repressors.
The only essential difference between inducible operons and repressible operons is whether the naked repressor or the repressor effector molecule complex is active in binding to the operator.
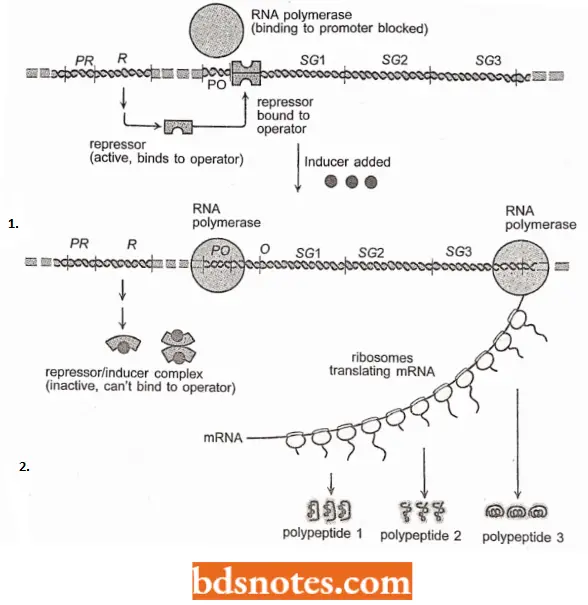
- In the case of an inducible operon, the free repressor binds to the operator, turning it off. When the effector molecule or the inducer (for example., allolactose in lac operon) is present, it binds to the repressor, that is, the repressor-inducer complex cannot bind to the operator.
- Thus, the addition of an inducer turns on (or induces) the transcription of the structural genes in the operon.
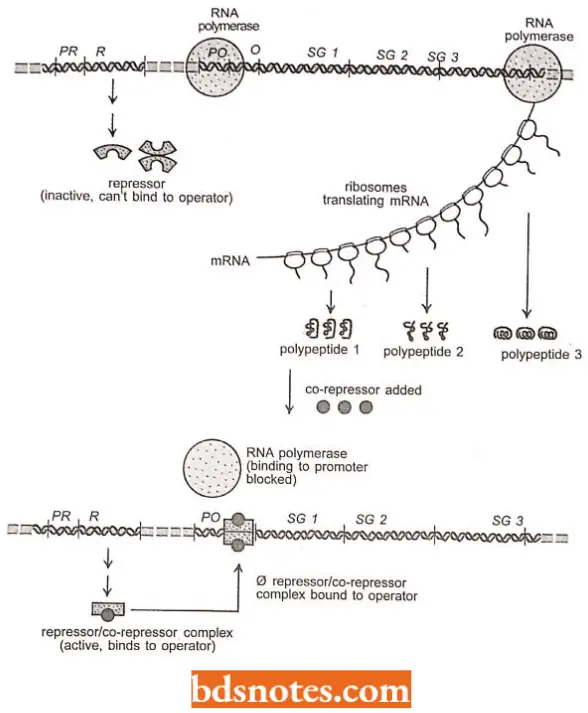
- In the case of a repressible operon (for example., trp operon), the situation is just reversed. The free repressor- cannot bind to the operator. Only the repressor-effector molecule (co-repressor) complex is active in binding to the operator.
- Thus, transcription of the structural genes in the repressible operon is turned on in the absence of and turned off in the presence of the effector molecule (co-repressor). Except for this difference in the operator, repressible operons are comparable.
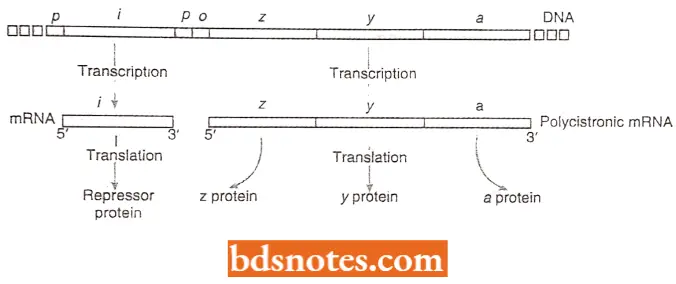
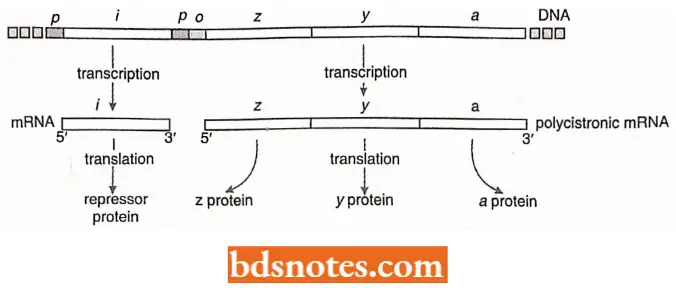
- A single mRNA transcript carries the coding information of an entire operon. Thus, the mRNAs of operons consisting of more than one structural gene are polygenic or polycistronic.
- For example, the tryptophan operon mRNA of E.coli is a huge macromolecule carrying the coding sequences that specify five different polypeptides because of their cotranscription, all the structural genes in an operon are coordinately expressed.
Examples of Operons
Lac Operons (Inducible System): Lactose (milk sugar, a disaccharide) is a β-galactoside that E. coli can use for energy and as a carbon source after it is broken down into glucose and galactose. The enzyme that performs the breakdown is β-galactosidase:
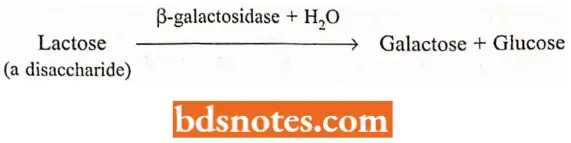
There are very few molecules of β-galactosidase enzyme in a wild-type E. coli cell grown in the absence of lactose. Within minutes after adding lactose to the medium, however, this enzyme appears in large quantities within the bacterial cell.
When the synthesis of β-galactosidase (encoded by the lacZ or z gene) is induced, the production of two additional enzymes is also induced, β-galactoside permease (encoded by the lacY or y gene) and β-galactoside acetyltransferase (encoded by the lac A or a gene).
The permease (enzyme) is involved in transporting lactose into the cell. The transferase is believed to protect the cell from the build-up of toxic products created by β-galactosidase (enzyme) acting on sugars other than lactose, the transferase prevents β-galactosidase from cleaving.
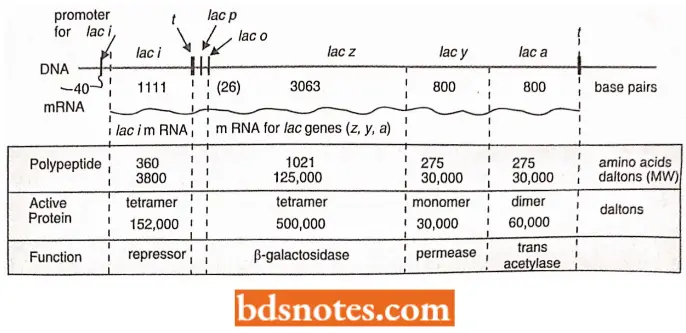
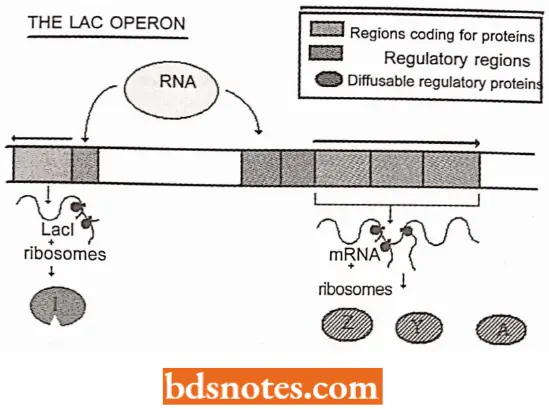
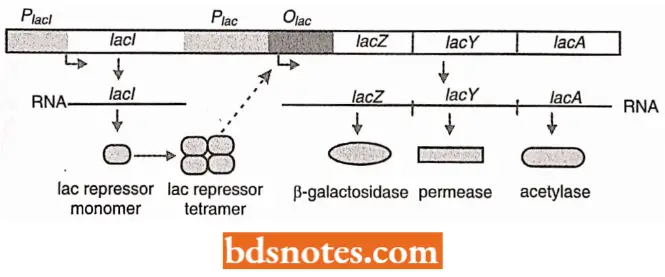
- The regulator gene. Not only are three lac genes (z, y, and a) induced together, but they are adjacent to one another in the E. coli chromosome; they are transcribed on a single polycistronic messenger RNA.
- Induction involves the protein product of another gene, the regulator gene, or gene (lac I). Although the regulator gene is located adjacent to the three other lac genes (i.e., three structural genes), it is an independent transcriptional entity.
- The regulator gene specifies a protein, the repressor, that interferes with the transcription of the genes involved in lactose metabolism.
- The operator. For the repressor protein to exert its influence over transcription, there must be a control element (receptor site) located near the beginning of the P-galactosidase (lacZ) gene.
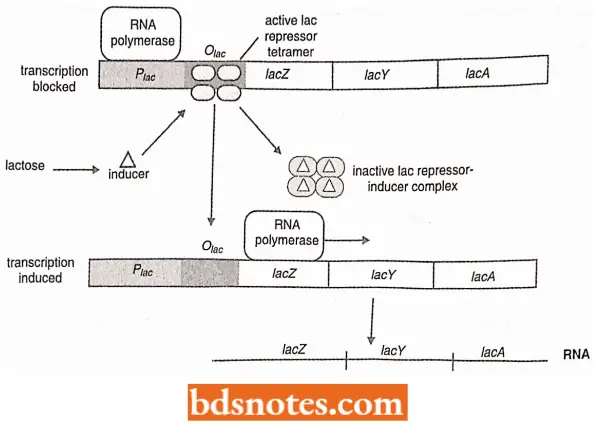
This control element is a region referred to as the operator, or operator site. the operator site is a sequence of DNA that the product of the regulator gene, the repressor, recognizes. When the repressor is bound to the operator, it interferes with RNA polymerase binding or prevents the RNA polymerase from achieving the open complex.
- In either case, transcription of the operon is prevented. The repressor is released when it combines with an inducer, a derivative of lactose called allolactose.
- Note that the promoter is not only recognized by RNA polymerase but also has elements near the initiation site of transcription. At this stage, an operon can be redefined as a sequence of adjacent genes all under the transcriptional control of the same promoter and operator.
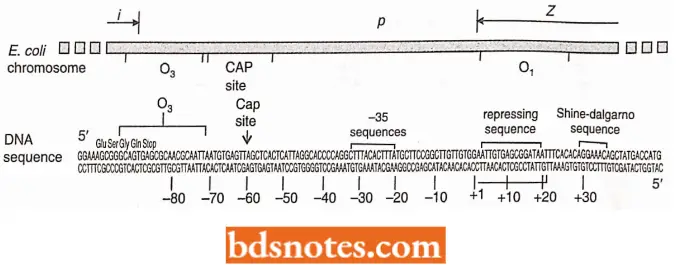
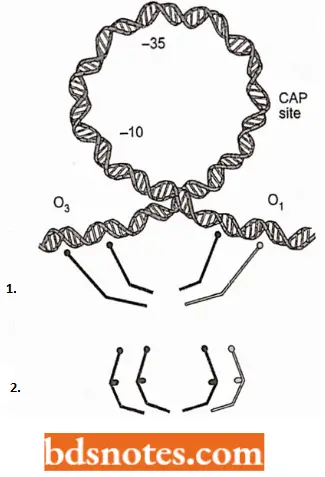
- The nucleotide sequence of the lac operator region. The operator is referred to as the primary operator, O1, centered at +11. Two other operator sequences have been found.
- One, O2, is centered at +412. The third operator, O3, overlaps the C-terminal end of the i gene and is centered at 82.
- The structure of the repressor and its interaction with the operator site was worked out recently with X-ray crystallography. The functional repressor is a homotetramer of the protein product of a gene; i.e., it is formed from four identical copies of the repressor protein.
- Since each operator site has two-fold symmetry, two repressor monomer proteins bind to each operator site. The monomer is shaped so that it fits into the major groove of the DNA to locate the exact base sequence of the operator; it then binds at the point through electrostatic forces.
- A tetramer can bind to two of the operator sites at the same time, presumably O1 and O3 or O1 and O2. In the process, the DNA is formed into a loop.
- Induction of the lac operon. Before the operon can be “turned on” to produce lactose utilizing enzymes, the repressor will have to be removed from the operator.
- The repressor is an allosteric protein; when it binds with one particular molecule, it changes the shape of the protein, which changes its ability to react with a second particular molecule.
- Here the first molecule is the inducer allolactose and the second molecule is the operator DNA. When allolactose is bound to the repressor, it causes the repressor to change shape and lose its affinity for operator sequences.
- With allolactose (inducer) bound to the repressor, the ability of the repressor to bind to the operator is greatly reduced, by a factor of 103. Since no covalent bonds are involved, the repressor simply dissociates from the operator. After the repressor is released from the operator, RNA polymerase can now begin transcription.
- The three structural genes of the lac operon are then transcribed and subsequently translated into their respective proteins. This system of control is very efficient.
- The presence of the lactose molecule permits transcription of the structural genes of the lac operon, which acts to break down the lactose.
- After all the lactose is metabolized, the repressor molecule returns to its original shape and can again bind to the operator. The system is “turned off”.
Lac Operon Mutants (Genetic Manipulations Of Lac Operon): Verification of the lac operon system came about through the use of mutants and partial diploids (merozygotes) of the lac operon well before DNA sequencing techniques had been developed.
- The structural (i.e., enzyme specifying) genes of the lac operon, z, y, and a, all have known mutant forms in which the particular enzyme does not perform its function. These mutant forms of the enzymes are z+, y+, and a+.
- Merozygotes or partial diploids in E. coli can be created through sexduction because some strains of E. coli have the lac operon incorporated into a factor.
- Since the F+ strain can pass the F‘ particle into the F– strain, lac operon diploids can be formed. By careful manipulation, various combinations of mutations can be looked at in a diploid state.
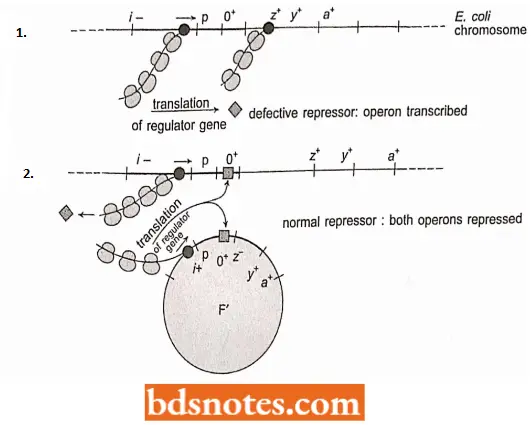
Constitutive Mutants are mutants in which the three lac operon structural genes are transcribed at all times, i.e., they are not turned off even in the absence of lactose. Constitutive production of enzymes can come about in several ways.
- A defective repressor, produced by a mutant regulator gene, will not turn the system off, nor will a mutant operator that will no longer bind the normal repressor.
- The regulator constitutive mutants are designated f; the operator constitutive mutants are designated Cf. Both types of mutants produce the same phenotype: constitutive expression of the three lac operon genes.
- When a new mutant is isolated, it is possible to determine whether it is caused by a regulator or operator mutation.
- For example, we can determine the exact location of a mutation on the bacterial chromosome by standard mapping techniques or, more recently, by DNA sequencing.
- Alternatively, the Jacob and Monod model predicts different modes of action for the two types of mutations.
- In merozygotes, a constitutive operator mutation affects only the operon it is physically a part of. Operator mutations are therefore called cis-dominant.
- However, a constitutive i-gene mutation, since it works through an altered protein, is recessive to a wild-type regulator gene in the same cell, regardless of which operon (chromosomal or F’ factor) the mutation is on.
- Constitutive regulator mutations are, therefore, trans-acting. (Note: If two mutations are on the same piece of DNA, they are in the cis configuration.
- If they are on different pieces of DNA, they are in the trans configuration). Transacting mutations usually work through a protein product that diffuses through the cytoplasm. C/sacting mutants are changes in recognition sequences on the DNA.
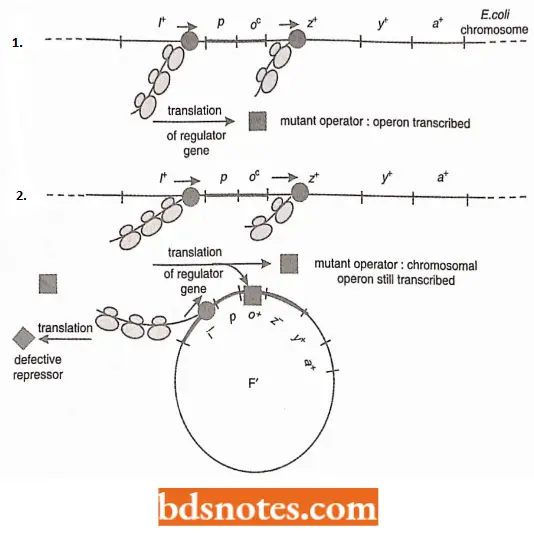
- Thus, when the bacterium has a regulator constitutive mutation (f); the cell has constitutive production of the operon.
- If the wild-type regulator is introduced in an F’ plasmid, the normal (i.e., inducible) phenotype is restored because the F’ i+ allele is dominant to the chromosomal mutation- the i+ gene regulates both the chromosomal and F’ operons.
- Hence, both operons are inducible. We do not need to be bothered about the other components of the F’ plasmid, because it carries a z–allele; only the activity of the chromosomal operon will be observed. When the chromosomal operon carries an operator constitutive mutation; the cell also has constitutive production of the operon.
- When a wild-type operator is introduced into the cell in an F’ plasmid, the cell still has the constitutive phenotype because the operator allele on the F’ plasmid does not control the bacterial operon; the lac operon bacterial chromosome will be continually transcribed.
- The chromosomal operon has a cls-domain operator mutation that has a constitutive phenotype. One should also note that only the bacterial chromosome determines the phenotype because the introduced F’ plasmid has a z–allele.
Other types of lac operon control mutations: Jacob and Monod’s operon model has been supported by some other types of mutations. For example, a super-repressed mutation was discovered. This mutation represses the operon even in the presence of large quantities of the inducer.
- Thus, the repressor seems to have lost the ability to recognize the inducer. The gene product is acting as a constant repressor, rather than as an allosteric protein.
- In an iS/i+ merozygote, both operons are repressed because the iS repressor binds to both operators. Another mutation reproduces much more of the repressor than normal and presumably represents a mutation of the promoter region of the I gene (see Tamarin, 2002).
- In 1966, W. Gilbert and B.MnHcr- Hill isolated the late repressor and thereby provided final proof of the validity of the method. M. Ptasline and his colleagues in 1992 isolated the repressor for phage λ operons.
Catabolite Repression Of Lac Operon (Glucose Effect)
An unusual feature of the lac operon and other operons that code for enzymes that catabolize certain sugars (e.g., arabinose, galactose) is that they are all repressed in the presence of glucose.
- That is, glucose is catabolized in preference to other sugars. This is called the glucose effect or catabolic repression.
- The mechanism of catabolic repressor involves cyclic AMP(cAMP). In eukaryotes, cAMP acts as a second messenger, an intracellular messenger regulated by certain extracellular hormones.
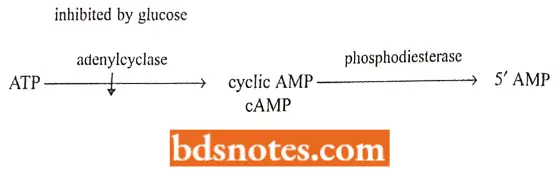
- Geneticists were surprised to discover cAMP in E. coli, where it works in association with another regulatory protein, the catabolite activator protein (CAP), to control the transcription of certain operons.
- When glucose is not present, cAMP combines with CAP, and the CAP-cAMP complex binds to a distal part of the promoter of operons with CAP sites (for example., the lac operon;).
- This binding increases the affinity of RNA polymerase for the promoter because, without the binding of the CAP-cAMP complex to the promoter, the transcription rate is very low.
- The uptake of glucose by E.coli cells causes the loss of cAMP from the cell, probably by inhibiting adenyl cyclase, and thus lowers the CAP-cAMP level.
- The transcription rate of operons with CAP sites will be reduced. The same reduction of transcription rates has been observed in mutant strains of E. coli when this part of the distal end of the promoter is deleted.
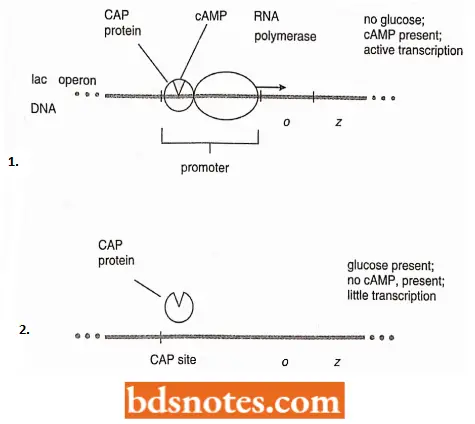
- The binding of CAP-cAMP to the CAP site causes the DNA to bend more than 90 degrees. This bending by itself, may enhance transcription, making the DNA more available to the RNA polymerase enzyme.
- During the process of initiation of transcription, the CAP is in direct contact with RNA polymerase.
- This was shown by photocrosslinking studies in which CAP was treated with a cross-linking agent that bound the subunit of RNA polymerase when irradiated with UV light. (Photocrosslinking is a technique used to determine which moieties (proteins, DNA) are nearby during a particular process).
- For the two proteins to cross-link, they must be in direct contact during the initiation of transcription. Catabolite repression is an example of positive regulation: The binding of the CAP-cAMP complex at the CAP site enhances the transcription rate of the transcriptional unit.
- Thus, the lac operon is both positively and negatively regulated; the repressor exerts negative control, and the CAP-cAMP complex exerts positive control of transcription.
TRP Operon: The catabolic operons or inducible operons are activated when the substrate that is to be catabolized enters the cell. Anabolic operons function in the reverse manner. They are turned off (repressed) when their end product accumulates beyond the needs of the cell.
- Two entirely different mechanisms seem to control the transcription of repressible operons. The first mechanism follows the basic scheme of inducible operons and involves the end product of the pathway.
- The second mechanism involves a secondary structure in messenger RNA transcribed from an attenuator region of the operon.
- Tryptophan synthesis. Tryptophan operon is an example of a repressible system. It contains the five genes that code for the synthesis of the enzymes that build tryptophan amino acid, starting from chorismic acid. It has a promoter operator sequence (p, o) as well as its regulator gene (trpR).
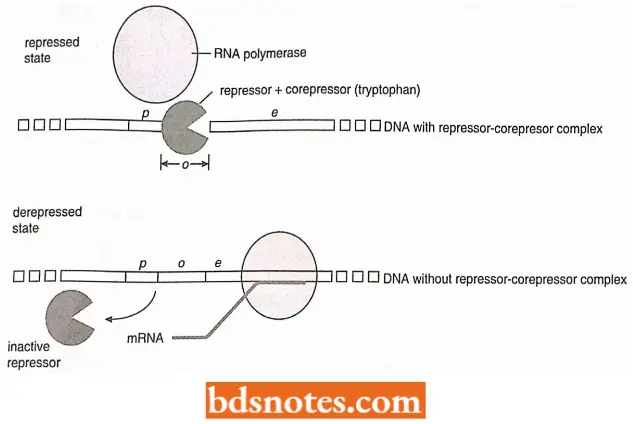
Operator Control: In trp popcorn, the product of the trpR gene is called a repressor. It is inactive by itself it does not recognize the operator sequence of the operon. The repressor only becomes active when it combines with tryptophan.
- Thus, when tryptophan builds up, enough is available to bind with and activate the repressor. Tryptophan is, thus, called a corepressor. The compressor-repressor complex then recognizes the operator, binds to it, and prevents transcription by RNA polymerase.
- After the available tryptophan in the cell is used up, the diffusion process causes tryptophan to leave the repressor, which then detaches from the trip operator. Since the transcription process no longer is blocked, so proceeds normally (i.e., the operon is now derepressed).
- Transcription continues until enough of the various enzymes are synthesized to again produce an excess of tryptophan. Some tryptophan molecules become available to bind to the repressor and make a functional complex, and the operon is again shut off.
- This process is repeated to ensure that tryptophan is being synthesized as needed. This regulation is modified, however, by the existence of the second mechanism for regulating repressible operons- attenuation.
Attenuator-controlled trp operon: Details of the second control mechanism of repressible operons have been explained primarily by C. Yanofsky and his colleagues (1984), who worked with the tryptophan operon in E. coli.
This type of person is controlled by its attenuator region and a similar system is also found in operons involved in the synthesis of other amino acids such as leucine and histidine. (Attenuation means “premature termination”).
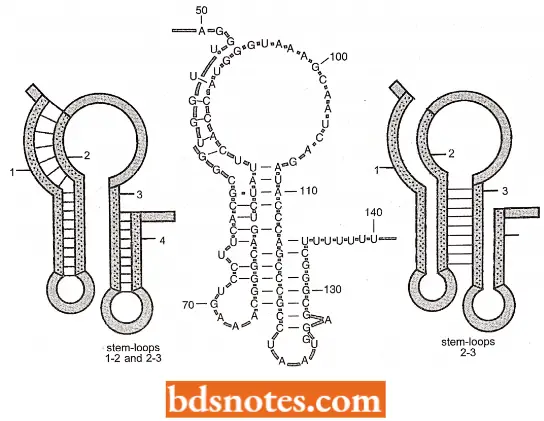
Leader Transcript: In the top operon, an attenuator region lies between the operator and the first structural gene. The messenger RNA (mRNA) transcribed from the attenuator region, termed the leader transcript, has been sequenced, revealing two surprising and interesting facts.
- Four subregions of the mRNA have base sequences that are complementary to each other so that three different stem-loop structures can form in the mRNA.
- Depending on conditions, regions 1-2 and 3-4 can form two stem-loop structures, or regions 2-3 can form a single stem-loop.
- When one stem-loop structure is formed, the others are acquired. As we will observe, the particular combination of stem-loop structures determines whether transcription continues.
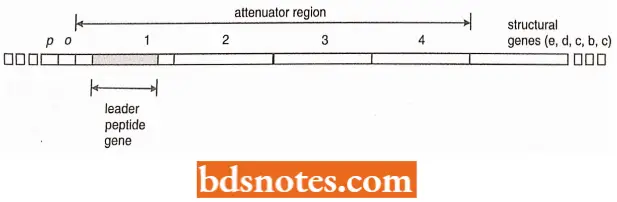
Leader peptide gene: Another fact has been obtained by sequencing the leader transcript and it has been found that there is a small gene coding information for a peptide from base 27 to 68. The gene for this peptide is called the leader peptide gene.
It codes for fourteen amino acids including two adjacent tryptophan. These adjacent tryptophan codons are critically important in attenuator regulation. The proposed mechanism for this regulation follows.

Assuming that the operator site is available to RNA polymerase, transcription of the attenuator region will begin. As soon as the 5′ end of the mRNA for the leader peptide gene has been transcribed, a ribosome attaches and begins translating this mRNA. Depending on the levels of amino acids in the cell, three different outcomes can take place.
Excess Tryptophan: If the concentration of tryptophan in the cell is such that abundant tryptophan-tRNAs exist, translation proceeds down that leader peptide gene. The moving ribosome overlaps regions 1 and 2 of the transcript and allows stem-loop 3-4 to form.
- This stem-loop structure is called a terminator or attenuator stem, causing transcription to be terminated. Note that stem-loop 3-4, the terminator stem, followed by a series of uracil (U)- containing bases, is a rho-independent transcription terminator.
- Hence, when existing quantities of tryptophan, in the form of tryptophan-tRNA, are adequate for translation of the leader peptide ‘gene’, the transcription is terminated.
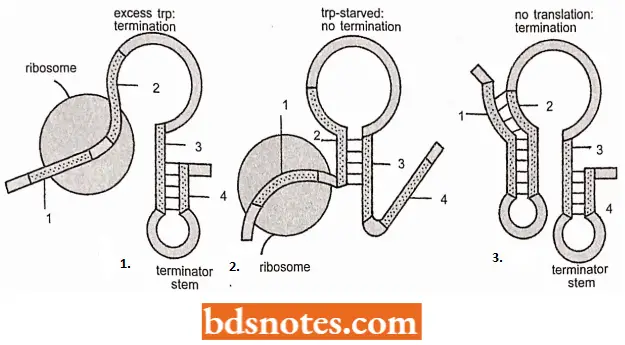
Tryptophan Starvation: If the quantity of tryptophan tRNA is lowered, the ribosome must wait at the first tryptophan codon until it acquires a Trp-tRNATrp. The stalled ribosome will permit stem-loop 2-3 to form, which precludes the formation of the terminator stem-loop (3-4).
- In this configuration, transcription is not terminated, so that eventually, the whole operon is transcribed and translated raising the level of tryptophan in the cell. The stem-loop 2-3 structure is referred to as the preemptor stem.
- This is important that the preemptor stem is not an independent transcription terminator and thus, without the rho protein present, will not terminate transcription.
General Starvation: A final configuration is possible. Here, no ribosome interferes with stem formation, and presumable stem-loops 1-2 and 3-4 (terminator) form. This configuration also terminates transcription because of the terminator stem.
- It is believed that this configuration occurs if the ribosome is stalled on the 5′ side of the trp codons, which happens when the cell is starved for other amino acids. Presumably, it makes no sense to manufacture tryptophan when other amino acids are in short supply.
- Hence, the cell can carefully bring up the levels of the various amino acids in the most efficient manner. The tryptophan operon in bacilli such as Bacillus subtilis, is also controlled by attenuation, but a secondary structure in the mRNA transport is induced by binding not the ribosome, but a trp RNA-binding attenuation protein (TRAP) (Antson et al, 1999).
The Ara Operon: The almost complete mechanism by which the lac and trp operons of E. coli are regulated, is known and supported by an extensive body of experimental data. However, the arabinose (ara) operon of E. coli exhibits much more complex patterns of regulation that are still not completely understood.
- In the lac and tip operons, the product of the regulator gene, the repressor, functions in a negative manner, turning off transcription of the operon.
- On the other hand, the catabolic activator protein (CAP) exerts a positive control over the lac operon by stimulating transcription of the operon.
- The major regulatory protein of the ara operon exhibits both negative and positive regulatory effects on the transcription of the structural genes of the operon, depending on the environmental conditions.
- Moreover, the regulatory components that control transcription of the ara operon include one element that acts from a distance of over 200 nucleotide pairs away from the promoter that it helps to control.
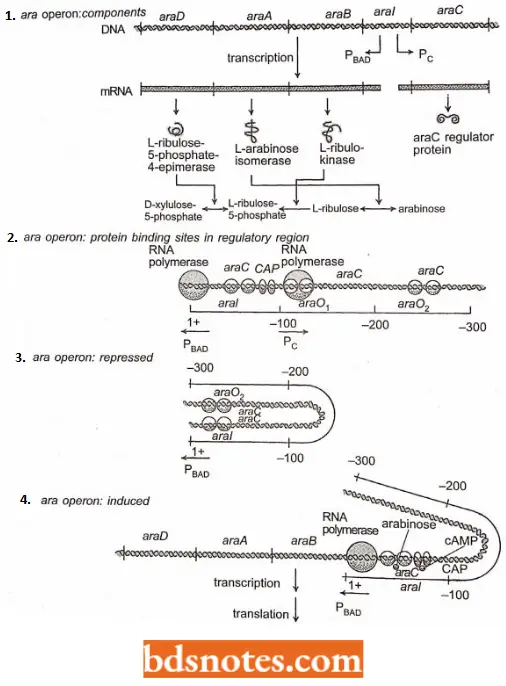
- The arabinose (ara) operon of E. coli contains three structural genes (araB, araA, and araD) that encode the three enzymes involved in the catabolism of arabinose. These three genes are cotranscribed on a single mRNA that is initiated at a promoter called PBad.
- Active transport of arabinose into cells is carried out by the products of genes araE, araF, and araG. These genes are located at sites quite distant from the araBAD operon of interest here and will not be further discussed.
- The major regulatory protein of the ara operon (i.e., the araCprotein) is produced from a transcript that is initiated at a promoter called Pc.
- The Pc promoter is only slightly over 100 nucleotide pairs away from PBAD, but the two promoters initiate transcription in opposite directions.
- The araC protein acts as a negative regulator (a repressor) of transcription of the araB, araA, and araD structural genes from the PBAD promoter in the absence of arabinose and cAMP. It acts as a positive regulator (an activator) of transcription of these genes from the PBAD promoter when arabinose and cAMP are present.
- Thus, depending on the presence or absence of the effector molecules arabinose and cAMP, the araC regulatory gene products may exert either a positive or a negative effect on the transcription of the araB, araA, and araD structural genes.
Since the operon is subject to catabolite repression like the lac operon and thus to positive control by CAP and cAMP, induction of the ara operon depends on the positive regulatory effects of two proteins, the araC protein and CAP.
- The binding sites for these two proteins and RNA polymerase all appear to be located in a region of the ara operon historically called oral (I for induction), located between the three structural genes of the operon and the regulatory gene (araC). ara Once geneticists working on the problem of regulation of the ara operon thought that all the binding sites for the araC regulatory protein and cAMP-CAP complex were in the oral region.
- The surprising discovery was that repression of the ara operon depended on the binding of ofaraC protein at a site called araO2 (O for operator, 2 because it was the second ara operator identified) located 211 nucleotide-pairs upstream (relative to the direction of the transcription from PBAD) from the araC protein binding site in oral. (Note: operator araO2– the first ara operator to be identified – controls the transcription of the araC regulator gene initiated at Pc).
- The currently accepted model for the repression of the ara operon is that the araC protein must bind (as a dimer) at both the aral site and the araO2 site and that these proteins then bind to each other to form a DNA loop.
- When the loop structure is formed, it must prevent or interfere with the binding of RNA polymerase at the adjacent promoter (PBAD) of the operon. In the presence of arabinose and cAMP, the ara operon is induced. Moreover, under these conditions, the ara C protein has been shown to become an activator of transcription of the operon.
- The arabinose- are protein complex and the cAMP-CAP complex must open the loop by binding at their aral sites. This, in turn, must permit RNA polymerase to bind at the PBAD site and initiate transcription of the ara structural genes.
Mode Of Working Of Gene Regulatory Proteins: Gene regulatory proteins must recognize specific nucleotide sequences embedded within their structure.
- It was originally thought that these proteins might require direct access to the hydrogen bonds between base pairs in the interior of the double helix to distinguish between one DNA sequence and another.
- It is now clear, however, that the outside of the double helix is studded with DNA sequence information that gene regulatory proteins can recognize without having to open the double helix.
Molecular Recognition: Gene regulatory proteins are found to contain structural motifs that read DNA sequences. Molecular recognition in biology generally relies on an exact fit between the surfaces of two molecules, and the study of gene regulatory proteins has provided some of the clearest examples of this principle.
- A gene regulatory protein recognizes a specific DNA sequence because the surface of the protein is extensively complementary to the specific surface features of the double helix in that region.
- In most cases, the protein makes a large number of contacts with the DNA, involving hydrogen bonds, ionic bonds, and hydrophobic interactions.
- Although individual contact is weak, the 20 or so contacts that are typically formed at the protein-helix interface add together to ensure that the interaction is both highly specific and very strong.
- DNA-protein interactions include some of the highest and most specific molecular interactions known in biology. DNA-protein interactions are of the following two types.
Helix-turn-Helix Motif: The first DNA-binding protein motif to be recognized was the helix-tum-helix. This was originally identified in bacterial proteins; but later on, this motif has been reported in hundreds of DNA-binding proteins from both eukaryotes and prokaryotes.
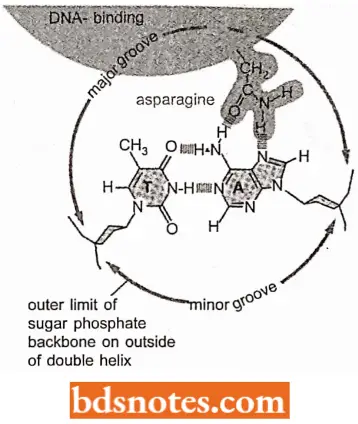
- This protein is constructed from two α-helices connected by a short extended chain of amino acids, which constitutes the “turn”. The two helices are held at a fixed angle, primarily through interactions between the two helices.
- The C-terminal helix is called the recognition helix because it fits into the major grooves of DNA; its amino acid side chains, which differ from protein to protein, play an important role in recognizing the specific DNA sequence to which the protein binds.
- Examples of helix-tum-helix proteins include tryptophan repressor, lambda cro, lambda repressor fragment, CAP fragment, etc.
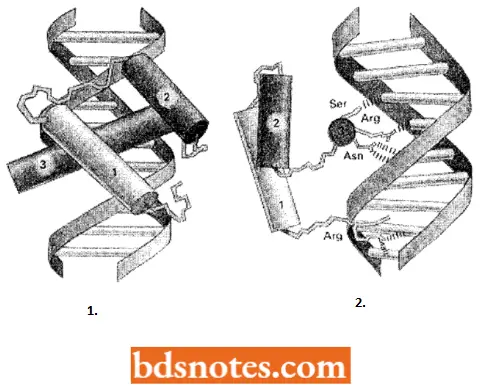
Homeodomain Protein: Soon after the discovery of gene regulatory proteins in bacteria, genetic analyses in the fruitfly Drosophila led to the characterization of an important class of genes, the homeotic selector genes, that play a critical part in coordinating fly development.
- Mutations in these genes cause one body part in the fly to be converted into another, showing that the proteins they encode control critical developmental decisions.
- When the nucleotide sequence of several homeotic selector genes of Drosophila and higher animals was determined in the early 1980s, each proved to contain an almost identical stretch of 60 amino acids that defines this class of proteins and is termed the homeodomain.
- When the three-dimensional structure of the homeodomain was determined, it was shown to contain a helix-tum-helix motif related to that of the bacterial gene regulatory proteins, providing one of the first indications that the principle of gene regulation established in bacteria is relevant to higher organisms as well.
- More than 60 homeodomain proteins now have been discovered in Drosophila alone, and homeodomain proteins have been identified in virtually all eukaryotic organisms that have been studied, from yeasts to plants to humans.
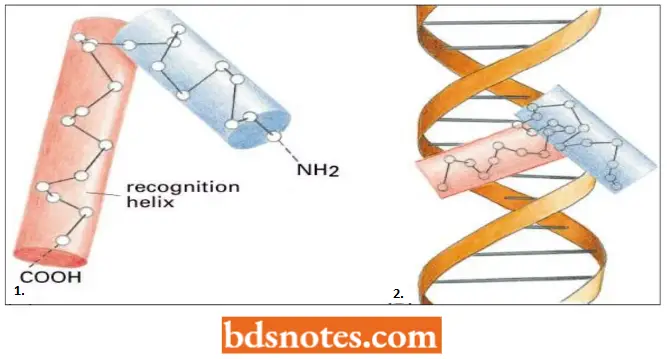
The structure of a homeodomain that is bound to its specific DNA sequence. Whereas the helix-tum-helix motif of bacterial gene regulatory proteins is often embedded in different structural contexts, the helix-turn-helix motif of homeodomains is always surrounded by the same structure, suggesting that the motif is always presented in the same way.
Other Types Of Motifs Of Gene Regulatory Proteins Include The Following:
- DNA-binding zinc finger motifs;
- β-sheet proteins;
- Leucine zipper motif; and
- Heterodimerization.
Translational Control
In prokaryotic gene regulation at the translation level, the lifetime of a mRNA molecule may be genetically determined. Enzymatic degradation of mRNA is from the 5′ to the 3′ end, i.e., the end of the RNA that is first synthesized is also the end that is first degraded.
- The average lifetime of many mRNA molecules of E. coli is only two minutes at 37°C. The specific nucleotide sequences at the 5′ end may influence its susceptibility to enzymatic digestion.
- Further, catabolic enzymes are denied access to the mRNA when the ribosomes are coated at their 5′ ends (i.e., in the case of polyribosomes).
- Hence, the lifetime of mRNAs may also be correlated with the number of free ribosomes available at any given moment to translate mRNA molecules.
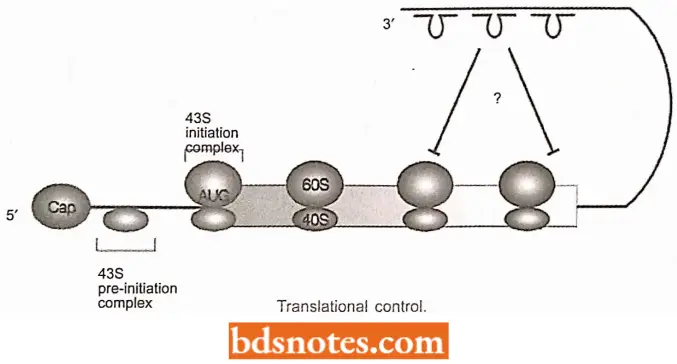
Bacteria vary their rates of protein synthesis by varying their ribosomal content rather than by varying the translational rate. Example: In the lactose system of E. coli, there are three structural genes under the control of a common operator locus determining the production of
- β- galactosidase,
- Galactoside permease and
- Galactoside acetylase.
These three proteins are produced in the respective ratios l.T/2:l/5, reflecting their respective locations relative to the 5′ (operator) end of the polycistronic mRNA in which they are coded (these differences are examples of translation regulations).
- Thus, there is a polarity gradient within the polycistronic mRNA that reduces the probability of cistron translation as a function of its distance from the 5′ end.
- It is hypothesized that ribosomes attach to different starting points (ribosome-binding sites) along the polycistronic mRNA at different rates as reflected by the amounts of the three proteins synthesized.
Post-translational control (Feedback Inhibition or End Product Inhibition): The expression of genes also can be regulated after proteins have been synthesized.
- This is called post-translational control of gene action. Feedback inhibition is a regulatory mechanism that does not affect enzyme synthesis, but rather inhibits enzyme activity.
- The end product of a biosynthetic pathway may combine loosely (if in high concentration) with the first enzyme in the pathway.
- This union does not occur at the catalytic site, but it does modify the tertiary structure of an enzyme and, hence, inactivates the catalytic site.
- This allosteric transition of protein molecule blocks its enzymatic activity and prevents the overproduction of end products and their intermediate metabolites.
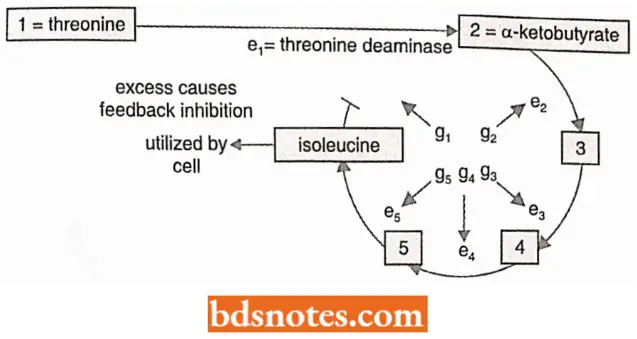
Example: The studies on isoleucine synthesis in E. coli (Umbarger, 1961) demonstrated that the addition of isoleucine (the end product of a five-step conversion of threonine) to a culture of the bacteria resulted in immediate blocking of the threonine-ÿisoleucine pathway.
- In the presence of added isoleucine, the cells preferentially use this exogenous end product (i.e., isoleucine) and their isoleucine synthesis ceases.
- Moreover, the production of each of the five enzymes is not interfered with, but the action of an enzyme responsible for the deamination of threonine to aketobutyrate is inhibited by the end product, isoleucine.
Operons Of Phage Lambda (Regulation Of Phage λ Life Cycles)
When a bacteriophage infects a bacterial cell, it has to express its genes in an orderly fashion; some gene products are needed early in infection, and other products are required during the later part of infection.
- Early genes usually control phage DNA replication; late genes usually determine phage coat proteins and the lysis of bacterial cells.
- A bacteriophage is considered most efficient if it expresses early genes first and late genes last in the infection process.
- Also, temperate phages have the option of entering into lysogeny with the cell, here, control processes determine which path is taken.
One generalization has been made regarding the phages that their genes are clustered into early and late operons, with separate transcriptional control mechanisms for each.
- Lysis: Bursting of a cell by the destruction of plasma membrane following an infection by a virus.
- Lysogenic: The state of a bacterial cell that has an integrated phage (prophage) in its chromosome.
- Lysogenic Bacteria: Bacteria harboring temperate bacteriophages.
- Temperate Phage: A phage (virus) that invades but may not destroy (lyse) the host
- (bacterial Cell): however, it may continue into the lytic cycle.
- Virulent Phage: A phage (vims) that destroys the host (bacterial) cell.
Phage λ (= lambda) is one of the best-studied bacteriophages. It has a double-stranded DNA (chromosome) of about 48,500 base pairs. Since it is a temperate phage, it can exist either vegetatively or as a prophage, integrated into the host chromosome.
- The life cycle of this phage is suitable for our analysis since its choice of pattern of life cycle is determined in an interesting and complex way.
- It provides a model system of operon controls. Here, complexity arises from having two conflicting life-cycle choices: one choice favors the lytic cycle and another choice prefers the nonlytic (i.e., lysogenic) phage of the life cycle.
- The expression of one of the two life-cycle alternatives, lysogenic or lytic cycles, depends on whether two repressors, Cl and cro, have access to the operator site. The Cl repressor acts to favor lysogeny: it expresses the lytic cycle and represses lysogeny.
- The operator sites, when bound by either Cl or cro, can either enhance or repress transcription. Certain other control mechanisms are involved in determining aspects of λ life cycle such as antitermination and multiple promoters for the same genes (see Ptashne and coworkers 1982; Ptashne, 1987, 1989).
Structure Of Phage λ Operons: Phage lambda (A.) shows a complex system of controls of both early and late operons, as well as controls for the decision of lytic infection versus lysogenic integration.
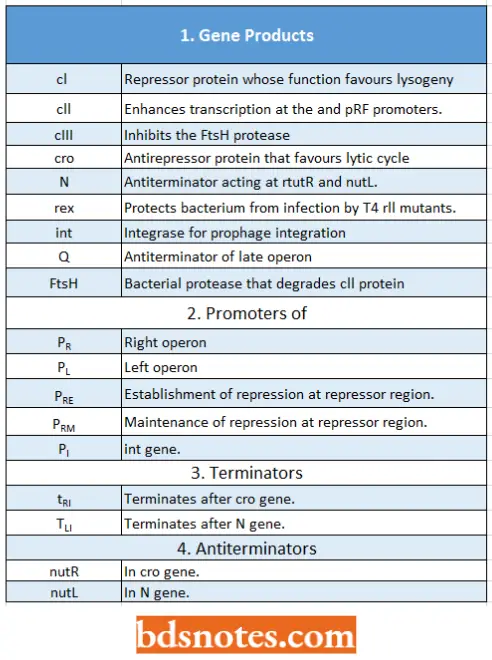
- The X genes are grouped into four operons: left and right, late, and repressor.
- The left and right operons contain the genes for DNA replication recombination and phage integration. The late operon contains the genes that determine phage head and tail proteins and lysis of the host cell. The sequence of events following phage infection is relatively well known.
- The genetic map of phage X is a circle, but the X chromosome has two linear stages in its life cycle. (Note: circular form of DNA is infective cellular form).
- In one linear form, its DNA is packed within the phage head, and in another linear form, the DNA of the phage becomes integrated into the host chromosome to form a prophage. These two linear forms of DNA do not have the same ends.
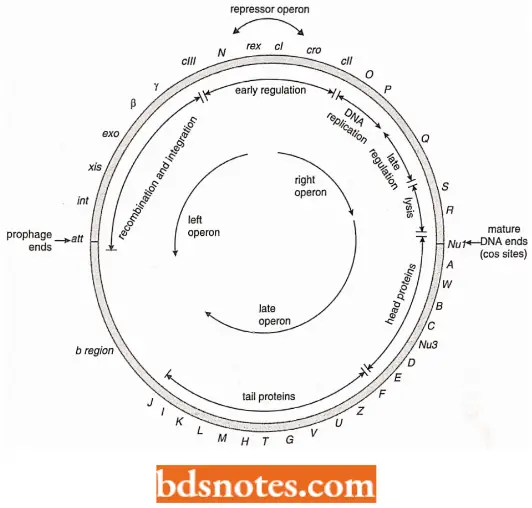
- The mature DNA which is packed within the phage heads before lysis of the cell, is flanked by cos sites. It results from a break in the circular map between the A and R loci. The prophage is integrated at the att site and the circular map is thus broken there at integration.
- The homologous integration sites on both X and the E.coli chromosome consist of a 15bp core sequence (called “0” in both), flanked by different sequences on both sides in both the bacterium and the phage.
- In the phage, the region is referred to as POP’ where P and P’ (P for phage) arc two different regions flanking the O core on the phage DNA.
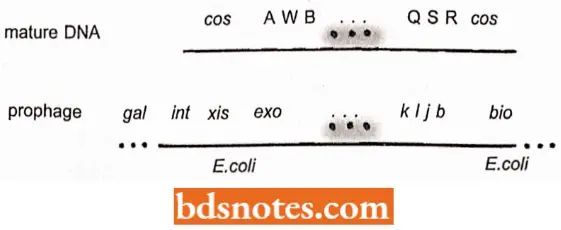
In the bacterium, the region is called BOB’ where B and B’ (B for bacterium) arc two different regions flanking the O E.coli chromosome.
- Integration, which is a part of the lysogenic life cycle, requires the product of the λ int gene, a protein known as integrase, and is referred to as site-specific recombination.
- Later excision of the prophage, during the introduction, when the phage leaves the host chromosome to enter the lytic cycle, requires both the integrase and the protein product of the neighboring xis gene, the excisional (enzyme).
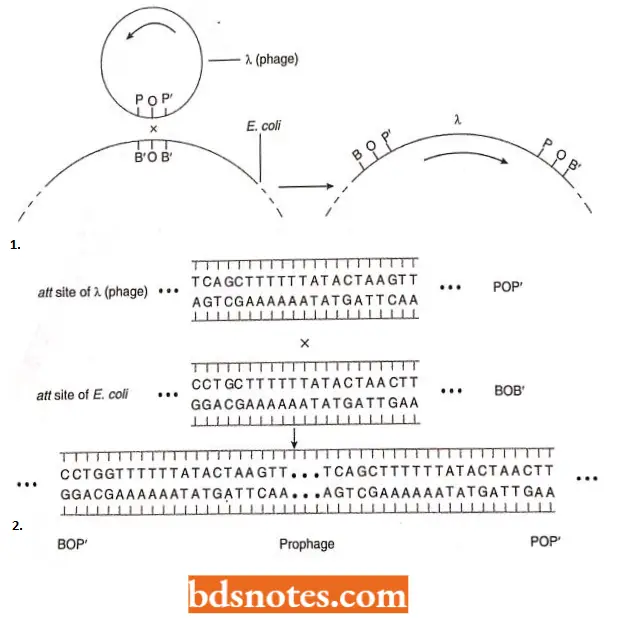
Following infection of the E.coli cell by a λ phage DNA circularizes, using the plementarity of the cos sites. Transcription begins, and within a very short time, the phage is producing virus progeny or entering the lysogenic cycle and integrating into the host chromosome.
Early And Late Transcription: When the phage first infects an E. coli cell, transcription of the left and right side operons is started at the left (PL) and right (PR) promoters, respectively:
- The N(left) and CRO (right) genes are transcribed and then translated into their respective proteins. Then, transcription stops on both operons at rho-dependent terminators (tRI, tLI).
- Transcription cannot continue until the protein product of the N gene is produced. This protein is called an antitermination protein.
- When it binds at sites upstream from the terminators, called nutL and nutR (nut stands for N utilization; L and R stands for left and right), the polymerase reads through the terminators and continues to transcribe the left and right operons. (Note: It is still not clear why antitermination has evolved here, it is believed that it gives the phage better control over the timing of events; see Tamarin, 2002)
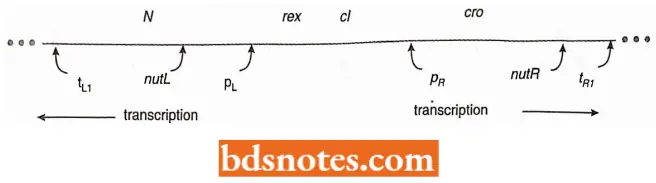
- Then transcription continues along the left and right operons through the ell and cIII genes. At a later stage, if the lytic response is followed by phage, the Q gene, which codes for a second antitermination protein in the right operon, has the same effect on the late operon as the N gene did on the two early operons.
- Thus, without the Q gene product, transcription of the late operon proceeds about two hundred nucleotides and then terminates.
- With the Q gene product, the late operon is transcribed. Hence, in phage λ, proteins that allow RNA polymerase to proceed past termination signals mediate general control of transcription.
- If only the previously described events were to take place, the lytic cycle pathway would always be followed. However, a complex series of events can occur in the repressor region that may lead to a “decision” to follow the lysogenic cycle instead.
Repressor Transcription: The cIII-gene product inhibits a host cell protease, called FtsH, that would normally break down the ell-gene product.
- The ell-gene product binds at two promoters, enhancing their availability to RNA polymerase just as (the CAP-cAMP product enhances the transcription of the lac operon.
- The cell protein binds to (lie promoters for cl transcription and in gene) and cl (repressor proteins) produced, favoring lysogeny, as well as the cm gene product, the antirepressor, which is a repressor of and therefore favors the lytic pathway, (cro stands for control of repression and other things.
- These mutants can only undergo lysis without the possibility of lysogeny. Normal λ infections produce turbid plaques, accounted for by lysogenic bacterial growth within the plaques).
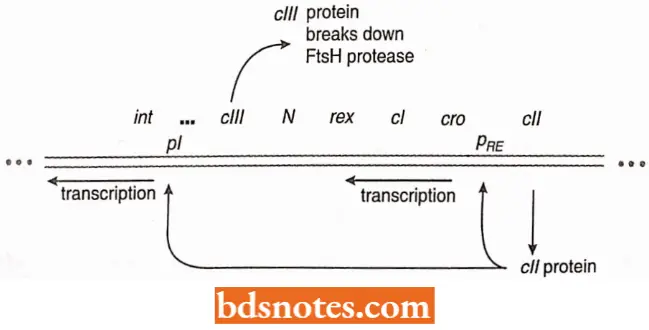
Maintenance of Repression: The cl gene, with the aid of the ell-gene product, is transcribed from a promoter known as PRE, (the RE stands for repression establishment;)
- Once cl is transcribed, it is translated into a protein called the X repressor, which interacts at the left and right operators, O, and OR of the left and right operons.
- When these operators are bound by cl protein, transcription of the left and right options (and therefore also the late operons) ceases. There are several subdivisions of the repression.
- First, lysogeny can be initiated because the int gene has been transcribed at the early stage of infection. Second, since ell and clll are no longer synthesized, cl transcription from the pRE promoter stops.
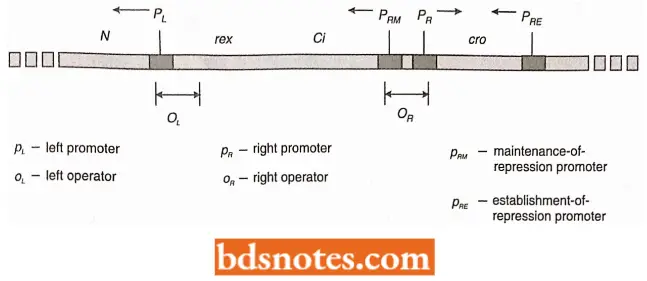
- However, PRM (RM stands for repression maintenance), allows low levels of transcription of the cl gene. The cl gene is found to control its concentration in the cell.
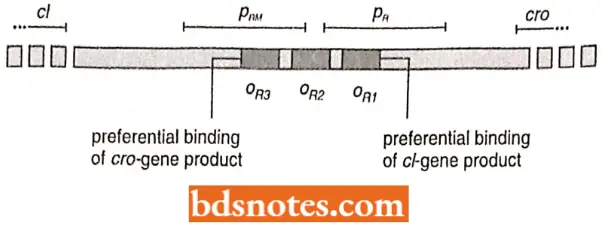
- When the right and left operators were sequenced, each was discovered to have three sites of repressor recognition.
- On the right operator, for example, the rightmost site (Ogl) was found to be most efficient at binding repressor. When the repressor was bound at this site, the right operon was repressed, and transcription of cl was enhanced (it is similar to the enhancement of transcription by the binding of CAP-cAMP at the CAP site in the lac operon).
- Excess repressor, when present, however, was also bound by the other two sites within OR. The above process results in the repression of the cl gene itself.
- Hence, maintenance levels of cl can be kept within a very narrow limit. A third subdivision of repression is the prevention of superinfection.
- That is, bacteria lysogenic for λ phage are protected from further infection by other λ phages because the repressor is already present in the cell.
- Thus, the excess of repressor controls new invading λ phages. (Note: for this situation, it is normally said that bacterial cells lysogenic for phage λ are immune from infection by additional λ phage).
- These bacteria are also protected from infection by T4 phage with all mutants. The rex-gene product, the product of the other gene in the repressor operon, controls the protection.
- Further, the promoters for maintenance and establishment of repression differ markedly in their control of repressor gene expression. When PRE is active, a veiy high level of repressor is present whereas PRM produces only a low level of repressor.
- The level of repressor is due to the length of the leader RNA transcribed on the 5′ side of the cl gene.
- The PRE promoter transcribes a very long leader RNA and is very efficient at translation of the cl region. In contrast, the PRM promoter starts transcription at the initiation codon of the protein. This leaderless mRNA is translated very’ inefficiently in Cl.
- The λ repressor is a dimer of two identical subunits. Each subunit is composed of two domains, or “ends”.
- The carboxyl- and amino-terminal ends are separated by a relatively open region which is susceptible to attack of protease enzyme.
- The alpha-helical parts of the amino-terminal ends interdigitate into the major groove of the DNA to locate specific sequences making up the left and right operator sequences. As described earlier in context with lac operon, the operator, OR1 OR2 and OR3 each have two-fold symmetry.
- The binding of the λ repressor in OR1 enhances the binding of another molecule or repressor into OR2. Together, they enhance PRM transcription, presumably through contact with polymerase. The repressors also block PR transcription.
Lysogenic versus Lytic Response: Now let us see how does λ phage turns toward the lytic cycle. In this context, the control is exerted by the cro-gene product, another repressor molecule that works at the left and right operators in a manner antagonistic to the way the Cl repressor works.
- In other words, using the right operator as an example, cro-gene product binds preferentially to the left of most of the three sites within oR and represses cl but enhances the transcription of cro.
- The cro-gene product can direct the cell toward a lytic response if it occupies the oR and oL sites before the λ repressor, or if the λ repressor is removed.
- From the point of view of phage λ, when would be a good time for the Cl repressor to be removed? From an evolutionary point of view, it is expected that a prophage might be at an advantage if it left a host’s chromosome and began the lytic cycle when it “sensed” damage to the host.
- One ofthe best ways to induce a prophage to enter the lytic cycle is to direct ultraviolet (UV) light at the host bacterium. UV light causes DNA damage and induces several repair systems. One, called SOS repair makes use of the protein products of the recA gene.
- Among the activities of this enzyme is to cleave the λ repressor in the susceptible region between domains. The cleaved repressor falls free of the DNA, making the operator sites available for the cro-gene product. The lytic cycle then follows. Various elements involved in phage λ infection have been tabulated.
Elements In Phage λ Infection:
- Under initial infection, the choice between lysogeny and lytic cycle depends primarily on the ell protein, which gauges the health activity of the host.
- After lysogeny is established, it can be reversed by the processes that inactivate the cl protein, indicating genetic damage to the bacterium (the SOS response), or an abundance of other hosts in the environment (zygotic induction).
- In zygotic induction, the lytic cycle is induced during conjugation, presumably when an Hfr cell sends a copy of the F– prophage into an F– cell. At that point, without a repressor present, the prophage can reassess whether to continue lysogeny or enter the lytic cycle.
Questions And Answers
Question 1. How can inducible and repressive enzymes of microorganisms be distinguished?
Answer: Both enzyme types are distinguished by studying the synthesis or lack of synthesis of the enzyme in cells grown on chemically defined media. If the enzyme is synthesized only in the presence of a certain metabolite or a particular set of metabolites, it is probably inducible. If it is synthesized in the absence but not in the presence of a particular metabolite or group of metabolites, it is probably repressible.
Question 2. Distinguish between
- Repression and
- Feedback inhibition is caused by the end product of the biosynthesis pathway. How do these two regulatory mechanisms complement each other to provide for the efficient regulation of metabolism?
Answer: Repression occurs at the level of transcription during enzyme synthesis. The end product, or a derivative of the end product, of a repressible system acts as an effector molecule that usually, if not always, combines with the product of one or more regulator genes to turn off the synthesis of the enzymes in the biosynthesis.
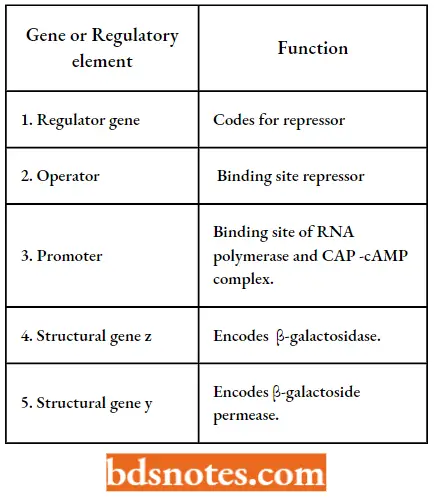
Question 3. In the lac operon of E. coli, what is the function of each of the following genes or sites:
- Regulator,
- Operator,
- Promoter,
- Structural genes z, and
- Structural gene y?
Answer:
Question 4. What would be the result of inactivation by mutation ofthe following genes or sites in the E. coli lac operon:
- Regulator;
- Operator;
- Promoter;
- Structural genes and
- Structural gene y?
Answer:
- Constitutive synthesis of the lac enzymes;
- Constitutive synthesis of the lac enzymes;
- Uninducibility of the lac enzymes;
- No β-galactosidase activity;
- No β -galactoside permease activity
Question 5. Of what biological significance is the phenomenon of catabolite repression?
Answer: Catabolite repression has evolved to assure the use of glucose as a carbon source when this carbohydrate is available, rather than less efficient energy sources.
Question 6. Describe the role of cyclic AMP in transcriptional control in E. coli.
Answer: Cyclic AMP, combined with CAP protein, attaches to CAP sites enhancing transcription of nonglucose, sugar metabolizing operon in E. coli. Glucose inhibits its formation by inhibiting adenyl cyclase.
Question 7. J. Beckwith isolated point mutations that were simultaneously uninducible for the lac, ara, mal, and gal operons, even in the absence of glucose. Provide two different functions that could be missing in these mutations.
Answer: One should have an idea about how these operons are controlled. Each operon requires an inducer and also the catabolic repression activation system. These mutants could be unable to make cAMP because the adenyl cyclase gene is defective. Alternatively, they could be making a defective catabolite-activating protein (CAP).
Question 8. Describe the interaction of the attenuator and the operator control mechanisms in the trp operon of E. coli under varying concentrations of tryptophan in the cell. How does attenuator control react to a shortage of other amino acids?
Answer:
- The E. coli trp operon functions as a normal repressible operon. In addition, attenuation, based on secondary structure and stalling of the ribosome on the leader transcript, can further prevent transcription.
- Attenuator control can be exerted based on other amino acids if their codons appear in the leader transcript, causing ribosome stalling.
Question 9. What are the three different physical forms that the phage λ chromosome can take?
Answer:
- The λ chromosome has one circular and two linear forms. The circular form is the infective cellular form.
- A break at one point (cos site) takes place during packaging into the phage head, and a break at another point forms the linear integrative prophage.
Multiple Choice Questions Answers
Question 1. In the operon concept, the regulator gene functions as
- Inhibitor
- Regulator
- Repressor
- All of these
Answer: 3. Repressor
Question 2. The operator gene of the lac operon is “turned on” when the lactose molecule binds to
- Operator gene
- Repressor gene
- Promotor site
- mRNA
Answer: 2. Repressor gene
Question 3. An example of an inducible operon is
- Lac-operon
- Try-operon
- Arginine operon
- Both 1 and 2
Answer: 1. Lac-operon
Question 4. How many promoters control the transcription of E.coli lac operon?
- One
- Two
- Three
- Four
Answer: 2. Two
Question 5. Which one of the following is not a component of the lac-operon model?
- Promoter
- Structural gene
- Primer gene
- Regulator gene
Answer: 3. Primer gene
Question 6. In E.coli, the presence of tryptophan amino acid causes
- Activation of operon
- Closure of operon
- Both of above
- None
Answer: 2. Closure of operon

Leave a Reply