Properties Of X-Ray
Question 1. how X-rays are produced. describe properties of X-rays in detail.
or
Define X-rays, write the properties of X-ray in detail.
or
Write short note on properties of X-ray.
or
Write short answer on properties of X-ray.
Answer. X-rays are defined as weight less package of pure energy (Photon) that are without electrical charge and that travel in waves along a straight line with a specific frequency and speed.
Read And Learn More: Oral Radiology Question And Answers
X-ray Principle
Fundamental principle of X-ray production is, X-rays are produced by the sudden deceleration or stoppage of rapidly moving stream of electrons at a positively charged metal target in a high vacuum tube.
Production of X-rays
- X-rays are produced in X-ray tube. When the X-ray machine is turned on, the electric current enter the control panel; via the plugged in cord and then to the tube head via extension wires in extension arms.
- The current is directed to the filament circuit through the step-down transformer, it reduces the 110–220 voltage to 3 to 5 volts.
- The filament circuit uses the 3 to 5 volts to heat the tungsten filament. The hot filament emits electron, this emission of electron from the cathode is known as thermionic emission. It forms the electron cloud around the cathode.
- When the exposure button is pushed the high voltage current is activated and the electron cloud is accelerated in X-ray tube from cathode to anode.
- Molybdenum cup of cathode directs the electron to the anode target in narrow beam.
- When the electron strikes the tungsten target, their kinetic energy is converted to X-ray energy. Less than 1 % of the energy is converted to X-rays, the remaining 99 % is lost as heat.
- The heat produced is carried away by copper stem and absorbed by the insulating oil in the tube head.
- The area where electron strike on tungsten (anode) is known as focus spot (Tungsten focus).
- Produced X-rays only exit from the X-ray tube via unleaded glass window portion of tube.
- X-rays travel through the unleaded glass window, the tube head seal, the aluminum discs, which filter the long wave X-rays from the beam.
- The size and shape of the X-ray beam is controlled by the lead collimator. X-ray beam then travels down the lead lines position indicating device and exit the tube head at the opening of position indicating device.
- X-rays produced in the X-ray tube vary in their energy and their wavelength , depending on how electrons interacted with tungsten atoms in anode. So kinetic energy of electrons is converted to X-ray photon either through Bremsstrahlung radiation or through characteristic radiation.
General (Bremsstrahlung Radiation or Breaking Radiation)
- The term refers to the sudden breaking of high speed electrons when they hit the tungsten target in the anode. 70 % of the X-rays are produced in this manner.
- If the electron hits the nucleus of the tungsten atom all its kinetic energy is converted into “High Energy X-ray Photon”.
- But most of the time, instead of hitting the nucleus, most electrons just miss the nucleus of the tungsten atom. When the electron comes close to the nucleus, it is attracted to the nucleus and slows down, consequently an X-ray photon is released. The electron that misses the nucleus continues to penetrate many such tungsten atoms producing many lower energy X-ray photons before it imparts all its kinetic energy.
- As a result-general-radiation consists of X-rays of many different energies and wavelengths. It is also known as continuous spectrum.
Characteristic Radiation
- It is produced when a high speed electron dislodges an inner shell electron from tungsten atom and leads to ionization of atom. Once the electron is dislodged remaining orbiting electrons are rearranged to fill the vacancy. This rearrangement causes loss of the energy which results in the X-ray photon, with the energy equal to the difference in two orbital energy states. So X-rays produced are known as characteristic radiation.
- This radiation accounts for very small part of X-rays which are produced in dental X-ray machine and occur at 70kVp and above.
Properties of X-rays
Properties of X-rays are of following types:
- Physical properties
- Chemical properties
- Biological properties
- Physiochemical properties
X-ray Physical Properties
- X-rays are electromagnetic radiation having a wavelength between 10 A° to 0.01A°.
- In free space, they travel in straight line.
- They travel with the same speed as that of visible light, i.e. 1,86,000 miles/sec.
- They are invisible to eye and cannot be seen, heard or smelt.
- They cannot be reflected, refracted or deflected by magnet or electric field.
- They show the properties of interference, diffraction and polarization similar to visible tight.
- They can produce an electrical field at right angles to their path of propagation and a magnetic field at right angles to the electric field and propagation.
- They do not require a medium for propagation.
- Penetration of X-rays can penetrate liquids, solids and gases. The degree of penetration depends upon the quality of the X-ray beam and also on intensity and wavelength of X-ray beam.
- Absorption X-ray are absorbed by matter, the absorption depends on the atomic structures of the matter and the wavelength of X-ray.
- Ionizing capability: X-rays interact with materials they penetrate and causes ionization.
- Fluorescence: When X-rays fall upon certain materials, visible light is emitted called fluorescence.
- X-rays has the property of attenuation, absorption and scattering.
- Heating effect.
X-ray Chemical Properties
- X-ray induces color changes of several substances or their solution, e.g. methylene blue gets bleached.
- X-ray bring about chemical changes in solution because X-rays produce highly active radical “OH” in water, which reacts with the solutes.
- X-ray can cause destruction of the fermenting power of enzymes.
X-ray Biological Properties
- The “excitation” property of X-rays are used in treatment of malignant lesions.
- X-rays also have a germicidal or bactericidal effect (sterilization and preservation of food).
- Somatic effect: This ranges from simple sun burn to severe dermatitis or to change in blood supply to malignancy.
- Genetic effect: Effect due to radiation-induced mutation of genes and chromosomes.
X-ray Physiochemical Properties
- X-ray can produce an image on a photographic film.
- PhotographicfilmwhenexposedtoX-rayradiation and then developed will be found blackened. Irradiation affects the silver salt in emulsion, so after developing, the radiograph metallic silver releases and films appears to be black.
Question 2. Write in detail about interaction of X-rays with matter.
Answer. As X-rays strike the matter such as tissue of the patient, photons interact with atoms in the absorber and have three possible fates, i.e.
- Coherent scattering (8%)
- Photoelectric effect (30%)
- Compton scattering (62%)
X-rays Coherent scattering
- It is also known as classical or elastic or Thompson scattering.
- Coherent scattering is the process by which radiation is deflected without losing energy.
- X-rays when pass close to an atom causes bound electrons to vibrate momentarily at a frequency which is equal to that of incident photon and the incident photon ceases to exist.
- The vibration causes electron to radiate energy in form of another X-ray photon of same frequency and energy as that in the incident beam. Usually, the second photon emitted is at an angle to the path of incidental X-ray.
- This interaction accounts for only 8% of total number of interactions in the dental examination.
- Coherent scattering is negligible in production of fog. This property is used to investigate internal molecular structure of materials by method of X-ray diffraction known as X-ray crystallography.
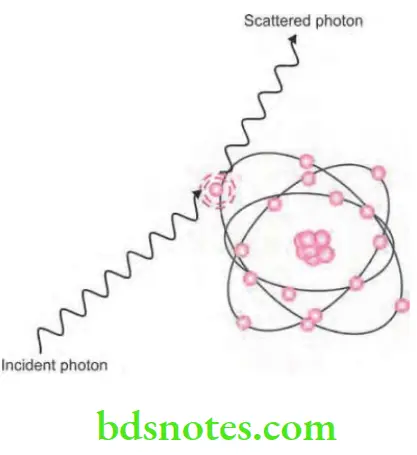
X-rays Photoelectric Effect
- It is the process of interaction of X-ray photon and the inner shell electron of atom.
- Inner shell electron is ejected which is now known as photoelectron. This will undergo further interactions.
- Energy of the photoelectron is equal to the energy of incident photon minus the ionization energy of inner shell electron.
- Vacancy present in the inner shell is filled by the outer shell electron leading to emission of characteristic radiation. Characteristic photons which are generated are of very low energy that they are absorbed inside the patient and do not fog the film.
- In this high energy, ejected photoelectrons behaves such as original high energy X-ray photon which undergo many similar interactions and ejecting other electrons as it pass through the tissues.
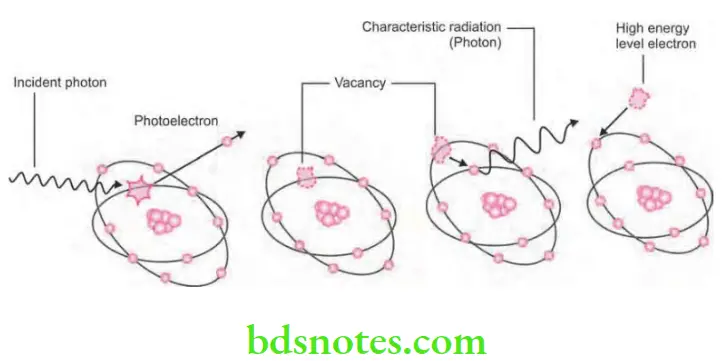
- So in the ejected high energy electrons which are responsible for majority of ionization interaction within the tissue and the possible resulting damage which is attributable to X-rays.
- Approximately 30% of photons which are absorbed from dental X-ray beam are absorbed by the photoelectric process.
- In diagnostic radiography, the characteristic radiation generated is of no significance as X-ray photons which are absorbed by the patients are of such a low energy that they are absorbed within the patient. So this is good for dentist as no scattered radiation is present but bad for the patient because of increased radiation absorption.
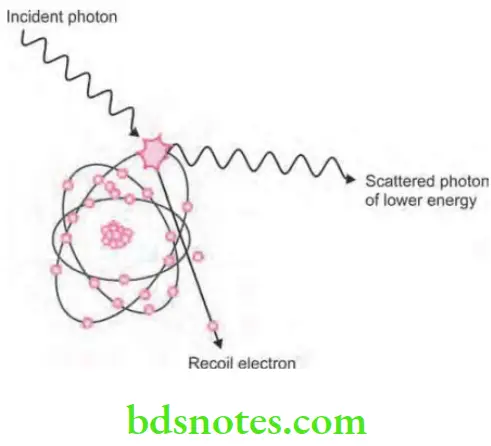
X-rays Compton scattering
- It is also known as inelastic scattering.
- Compton scattering is the interaction of photons with the free or loosely bound outer shell electrons, i.e. it is an absorption and scattering process predominating with higher energy photons.
- X-ray photon interacts with the outer shell electron of tissue atom and get ejected which is known as Compton recoil electron, with some energy of incoming photon this means there is some absorption.
- Ejected electron undergoes further ionizing interaction inside the tissues.
- Remainder of incoming photon energy is deflected or scattered from original path as scattered photon.
- Scattered photon then undergo further Compton interaction inside the tissues; photoelectric interaction inside the tissues and escape from the tissues.
- Photons which escaped from the tissues form scatter radiation in clinical era.
- Approximately, 62% of photons are absorbed from dental X-ray beam by this process.
- Importance of photoelectron and Compton absorption in diagnostic radiography relate to the difference in the way, photon are absorbed in various anatomic structures.
- The probability of both photoelectric and Compton interactions of photons with matter is more probable in hard tissues than in less mineralized soft tissues, there are more photons in the beam exiting the patient after traversing soft tissue, than through hard tissue. As a consequence, a radiograph readily distinguishes between many tissues including enamel, dentine, bone and soft tissue.

Leave a Reply