Pharmacokinetics
Question 1. Describe Various Factors That Affect The Process Of Absorption Of A Drug When It Is Given Orally?
Or
Write On Factors Influencing Git Absorption Of Drug.
Or
Describe The Various Factors Affecting Absorption Of Drugs.
Answer:
Various Factors Affecting Absorption Of Drugs
Absorption is the movement of a drug from its site of administration into circulation.
The factors affecting absorption when the drug is given orally:
- Physicochemical properties of the drug
- Physical state: The drug in liquid form is better absorbed than in solid form.
- Lipid-soluble and unionized form: The lipid-soluble and unionized form of the drug is better absorbed as compared to a water-soluble and ionized form of the drug.
Read And Learn More: Pharmacology Question And Answers
-
-
- Particle size: Drugs having small particle sizes are absorbed better as compared to large particle-sized drugs.
- Disintegration time: It is the time taken for a tablet or capsule to break up into small particles, variation in disintegration time affects bioavailability.
- Dissolution time: Dissolution time is the time taken by the particles to go inside the solution. As shorter the time better is the absorption.
- Formulation: Substances that are pharmacologically inherited such as lactose, starch, calcium sulfate, and gum are added as binding agents. These may affect the absorption of drugs.
-
- Route of drug administration: The drug administered through the parenteral route directly enters the circulation and bypasses the process of absorption.
- pH and ionization: Strongly acidic and strongly basic drugs remain ionized at all pH, so these drugs are poorly absorbed.
- Food: The presence of food inside the stomach interferes with the absorption of some of the drugs. Such drugs should be taken empty stomach.
- Presence of other drugs: Administration of two or more drugs can affect their absorption.
- Area of absorbing surface: Normally drugs are better absorbed in the small intestine due to their large surface area. If the gut is resected there is a decrease in the absorption of drugs due to reduced surface area.
- Gastrointestinal and other diseases: In gastroenteritis, peristaltic movement increases which decreases drug absorption. If achlorhydria is present absorption of iron from the gut is decreased.
Question 2. What Is The Concept Of Bioavailability? Describe The Factors Which May Affect The Bioavailability Of Drugs? Illustrate Your Answer With Suitable Examples.
Or
Describe The Bioavailability Of Drugs.
Or
Define Bioavailability And Describe Factors Influencing It.
Or
Write A Short Note On Bioavailability.
Answer:
Concept Of Bioavailability
Bioavailability is the measure of the fraction of an administered dose of a drug that reaches systemic circulation in the unchanged form that reaches the systematic circulation in the unchanged form.
- When we give a drug orally, first it is absorbed into the portal circulation and reaches the liver. Here some of the drugs may be metabolized (first-pass metabolism or pre-systemic metabolism) and the rest of the drug reaches the systemic circulation.
Thus absorption and first-pass metabolism are two important determinants of bioavailability. - Bioavailability by IV route is 100%.
- It can be calculated by comparing the AUC (area under plasma concentration-time curve) for the IV route and for that particular route. It can also be calculated by comparing the excretion in urine.
- Bioavailability determines the dose of a drug as compared to its intravenous dose.
It is clinically significant in the following situations:- With drugs having low safety margins, i.e. digoxin
- A precision action/effect of the drug is desired, i.e. oral hypoglycemic.
Factors Which May Affect Bioavailability
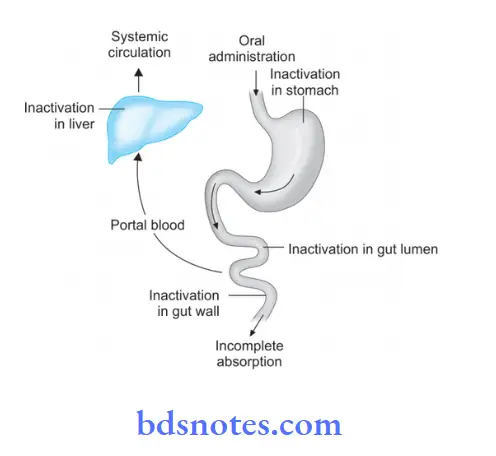
- Absorption: Incomplete absorption of drugs from GIT results in less bioavailability.
- Metabolism: Metabolism of the drug during the passage through the gut wall or liver decreases bioavailability.
- Rate of dissolution: Differences in bioavailability may arise due to variations in dissolution.
- Particle size: Reduction in particle size increases the rate of absorption, for Example. bioavailability of drug injected IV is 100% like noradrenaline but is frequently lower in drugs given orally.
Factors Affecting Bioavailability
- Disintegration and dissolution of time: Oral drugs have to disintegrate to be absorbed and then dissolved in gastrointestinal fluids. Liquids are absorbed faster than solids. Water-soluble drugs like aspirin reduce bioavailability.
- Formulation: Inert substances used with drugs as diluents like starch has slow absorption and reduce bioavailability.
- Particle size: Small particles are more easily absorbed than bigger particles and there is better bioavailability.
- Lipid solubility: Lipid-soluble drugs are absorbed faster and they are more bioavailable.
- PH and ionization: Ionized drugs are poorly absorbed compared to unionized drugs. Acidic drugs remain unionized in an acidic medium and are rapidly absorbed, for Example. aspirin, barbiturates.
- Area and vascularity of the absorbing surface: The larger the surfaces are for absorption and more vascularity, there will be more absorption and bioavailability.
- Gastrointestinal motility: Faster gastric emptying time ensures passage of the drug to the intestine is fast and more absorption. Intestinal motility as in diarrhea reduces absorption and the amount of drug available is reduced.
- Presence of food: Delays gastric emptying and delays absorption.
- Metabolism: Some drugs are degraded in the gastrointestinal tract and bioavailability becomes zero.
- Diseases: Bioavailability is increased in patients with liver disorders due to a reduction in hepatic metabolism.
Question 3. Write A Note On The Plasma Half-Life Of The Drug.
Answer:
Plasma Half-Life Of Drug
The plasma half-life of a drug is the time taken for its plasma concentration to be reduced to half of its original value.
- If metabolism is more, the half-life is less, and vice versa.
- Half-life is a secondary pharmacokinetic parameter derived from two primary parameters, i.e. volume of distribution and clearance.
- Plasma half-life determines:
- Dosing interval or frequency of drug administration. Drugs having shorter half-lives are administered more frequently compared to those having long half-lives.
- Time required to achieve the steady state plasma concentration. It takes 4 to 5 half-lives for a drug to reach a steady state.
Significance Of Plasma Half-Life
- It tells about the time to achieve steady-state plasma concentration.
- Knowing the half-life helps the prescriber decide whether or not to initiate treatment with a loading dose. Drugs with very long half-lives when given for acute conditions need to be given in loading dose.
- It tells about the time for which the therapeutic action of the drug is likely to be present.
- Alteration in half-life is expected in diseases affecting the liver and kidney function.
Types Of Plasma Half-Life
There are two types of plasma half-life:
1. Alpha phase:
This is due to the distribution of drugs. It is divided into Alpha 1 and Alpha 2:
- Alpha 1 is due to the distribution of the drug into the highly vascular organ.
- Alpha 2 is due to the distribution of the drug into less vascular organs.
2. Beta phase:
This is due to the elimination of drugs. The drugs are eliminated by:
- First-order kinetics: It remains constant.
- Zero-order kinetics: It increases with dosage.
Plasma half-life t½ = In 2/K where
- In 2 is the natural logarithm of 2
- K is the elimination rate constant of the drug.
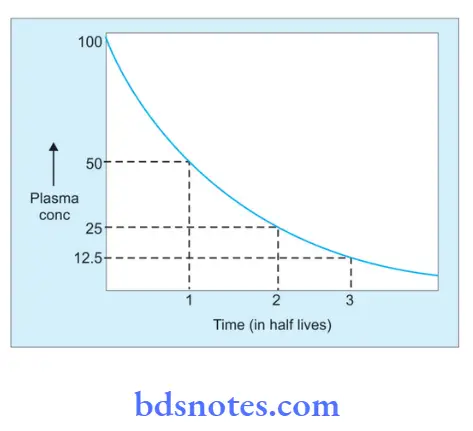
When a drug is given by constant IV infusion, initially the plasma level rises, it reaches a steady state and when the infusion is stopped this level starts declining.
Elimination of the drug from plasma is 50% in one half-life, 75% (50 + 25) in two half-lives, 87.5% (50 + 25 + 12.5) in three half-lives, and so on.
The same is true for rising plasma concentration also, i.e. with constant IV infusion, in one half-life the plasma concentration is half of the steady state, and in two half-lives, it is 75%, and so on.
Question 4. Describe The Process By Which Kidney Removes The Drug From The Body? How The Presence Of Other Drugs May Alter This Process? Illustrate Your Answer With an Appropriate Example.
Answer:
The Process Removes The Drug From The Body
The process of removal of the drug through the kidney involves:
- Filtration: Drugs are filtrated through the glomerulus along with other plasma constituents.
- Tubular reabsorption: This depends on the lipid solubility and ionization of the drug. If reabsorption is increased the urine become alkaline in barbiturate poisoning and acidifies in morphine poisoning.
- Tubular secretion: Acidic drugs like penicillin probenecid and basic drugs like quinine are secreted in the proximal tubules.
Some drugs alter these processes like:
- Probenecid reduces the excretion of penicillin, prolonging the duration of the effect.
- Probenecid also increases the excretion of uric acid in the patient with chronic gout.
- Salicylates block the tubular secretion of methotrexate.
Question 5. Give A Pharmacological Explanation For, When Creatinine Clearance Is Poor, Certain Drugs Are Not Used Or Used Very Cautiously?
Answer:
Pharmacological
Creatinine clearance is a reliable measure of glomerular filtration rate (GFR). Reduced GFR decreases the total rate of delivery of solute into the glomerular filtrate.
As kidneys are responsible for the excretion of all water-soluble drugs, the plasma concentration of such substances gradually increases with a decrease in GFR.
This problem is especially worse for all those substances which are not secreted by the renal tubules, hence their rate of excretion entirely depends on GFR.
Question 6. Explain The Methods Of Drug Absorption And Factors Affecting It.
Answer:
Methods Of Drug Absorption And Factors Affecting
The transfer of a drug from its site of administration to the blood so that it becomes available for distribution into various tissues is known as the absorption of drugs.
The transfer of drugs either into the blood or from the blood into the tissues takes place either by passive diffusion or by active absorption.
- Passive diffusion: In this, the rate of transfer of drug is proportional to the concentration gradient, on the two sides of the membrane. i.e. the drug moves from higher to lower concentration, till an equilibrium is reached. Water soluble drugs of small molecular size cross the membrane through the pores during the bulk flow of water this is known as filtration. Lipid-soluble unionized drugs pass through the membrane, the rate of transfer being proportional to the lipid-water partition coefficient of the drug. This process does not require energy and is nonsaturable.
- Active transport: Drugs that resemble endogenous substances in the chemical structure are absorbed by active transport. The characteristics of this process are:
- It can occur against a concentration gradient.
- It requires energy.
- A specific carrier is involved in the transport.
- The process is saturable.
- It can be inhibited competitively by the agents that resemble the transport of drugs in chemical structure.
- Pinocytosis: This involves the invagination of a part of a cell membrane by the extracellular constituents forming a vesicle.
This is then released within the cell or extruded from the other side of the cell membrane by exocytosis. It is important in the transport of some macromolecules.
Factors Affecting The Drug Absorption
Various Factors Affecting Absorption Of Drugs:
Absorption is the movement of a drug from its site of administration into circulation.
The factors affecting absorption when the drug is given orally:
- Physicochemical properties of the drug
- Physical state: The drug in liquid form is better absorbed than in solid form.
- Lipid-soluble and unionized form: The lipid-soluble and unionized form of the drug is better absorbed as compared to a water-soluble and ionized form of the drug.
- Particle size: Drugs having small particle sizes are absorbed better as compared to large particle-sized drugs.
- Disintegration time: It is the time taken for a tablet or capsule to break up into small particles, variation in disintegration time affects bioavailability.
- Dissolution time: Dissolution time is the time taken by the particles to go inside the solution. As shorter the time better is the absorption.
- Formulation: Substances that are pharmacologically inherited such as lactose, starch, calcium sulfate, and gum are added as binding agents. These may affect the absorption of drugs.
- Route of drug administration: The drug administered through the parenteral route directly enters the circulation and bypasses the process of absorption.
- pH and ionization: Strongly acidic and strongly basic drugs remain ionized at all pH, so these drugs are poorly absorbed.
- Food: The presence of food inside the stomach interferes with the absorption of some of the drugs. Such drugs should be taken empty stomach.
- Presence of other drugs: Administration of two or more drugs can affect their absorption.
- Area of absorbing surface: Normally drugs are better absorbed in the small intestine due to their large surface area. If the gut is resected there is a decrease in the absorption of drugs due to reduced surface area.
- Gastrointestinal and other diseases: In gastroenteritis, peristaltic movement increases which decreases drug absorption. If achlorhydria is present absorption of iron from the gut is decreased.
Question 7. Explain The Pattern Of Drug Distribution And The Factors Affecting It.
Answer:
Pattern Of Drug Distribution And Factors Affecting
Once a drug has gained access to the bloodstream, it gets distributed to other tissues that initially had no drug, the concentration gradient being in the direction of plasma to tissues.
The extent and pattern of distribution of a drug depend on its:
- Lipid solubility
- Ionization at physiological pH (a function of its pKa)
- The extent of binding to plasma and tissue proteins
- Presence of tissue-specific transporters
- Differences in regional blood flow.
Movement of the drug proceeds until an equilibrium is established between the unbound drugs in the plasma and the tissue fluids.
Subsequently, there is a parallel decline in both due to elimination.
The apparent volume of distribution (V) presumes that the body behaves as a single homogeneous compartment with volume (V) into which the drug gets immediately and uniformly distributed
\(\mathrm{V}=\frac{\text { Dose administered IV }}{\text { Plasma concentration }}\)
So as per this formula, if 1000 mg of the drug is injected IV, it produces a steady-state plasma concentration of 50 mg/L the apparent volume of distribution is 20 L.
The drug does not actually distribute into 20 L of body water, with the exclusion of the rest of it, this is only an apparent volume of distribution.
It can be defined as “the volume that would accommodate all the drug in the body if the concentration throughout was the same as in plasma”.
Thus, it describes the amount of drug present in the body as a multiple of that contained in a unit volume of plasma.
1. Factors Affecting Drug Distribution
The following are the factors governing drug distribution:
- Lipid–water partition coefficient of drug
- pKa value of the drug
- Degree of plasma protein binding
- Affity for diffrent tissues
- Fat-lean body mass ratio
- Diseases like congestive heart failure, uremia, cirrhosis
Lipid Solubility
Fat-lean body mass ratio—highly lipid-soluble drugs get distributed to the adipose tissue.
If the ratio is high, the volume of distribution for such a drug will be higher; fat acts as a reservoir for such drugs.
Lipid soluble drugs are more likely to cross the blood vessel wall and have more volume of distribution.
Molecular Size
Drugs with high molecular weight (For example. heparin) or extensively bound to plasma protein (For example. warfarin) are largely restricted to the vascular compartment, hence their apparent volume of distribution is low.
Affinity for Different Tissues
High lipid-soluble drugs get initially distributed to organs with high blood flow, i.e. brain, heart kidney, etc.
Less vascular but more bulky tissues take up drug plasma concentration falls and the drug is withdrawn from high-perfused sites.
The extent of the Binding of Drugs to Plasma Proteins
Most drugs possess a physicochemical affinity for plasma proteins and get reversibly bound to these.
Acidic drugs are generally bound to plasma albumin and basic drugs to α1 acid glycoprotein.
Clinical Importance Of Plasma Protein Binding
- The drug enters circulation and binds to plasma protein. The bound form, i.e. pharmacologically inactive form acts as a ‘temporary store’ of the drug and the free form is pharmacologically active.
- Plasma protein binding leads to drug absorption.
- Drugs that are highly bound to plasma proteins consist of a low volume of distribution.
- Plasma protein binding delays the metabolism of drugs.
- The bound form is not available for filtration at the glomeruli, hence excretion of highly plasma protein-bound drugs is delayed.
- Highly protein-bound drugs consist of a longer duration of action, for Example.
sulphadiazine is less plasma protein bound and has a duration of action of 6 hours while sulphadoxine is highly plasma protein bound and has a duration of action of one week. - In case of poisoning, highly plasma protein-bound drugs are difficult to remove via hemodialysis.
- In disease states like anemia, renal failure, chronic liver diseases, etc. plasma albumin levels are low. So, there will be an increase in the free form of the drug, which can lead to drug toxicity.
- Plasma protein binding can cause displacement interactions. More than one drug can bind to the same site on plasma protein.
The drug with higher affinity will displace the one having lower affinity and may result in a sudden increase in the free concentration of the drug with lower affinity.
2. Presence Of Tissue Barriers
Blood-Brain Barrier
The capillary boundary that is present between the blood and brain is called the blood-brain barrier (BBB).
In the brain capillaries, the endothelial cells are joined by tight junctions.
Only the lipid soluble and unionized form of drugs can pass through BBB and reach the brain, for Example. barbiturates, diazepam, volatile anesthetics, amphetamine, etc.
Lipid-insoluble and ionized particles do not cross the BBB, for Example. dopamine and aminoglycosides.
Pathological states like meningitis and encephalitis increase the permeability of the BBB and allow the normally impermeable substances to enter the brain.
Example. penicillin G in normal conditions has poor penetration through BBB, but its penetrability increases during meningitis and encephalitis.
Placental Barrier
Certain drugs administered to the pregnant woman can cross the placenta and affect the fetus/newborn, for Example. anesthetics, morphine, corticosteroids, etc.
Quaternary ammonium compounds, for Example. d-tubocurarine (d-TC) and substances with high molecular weight like insulin cannot cross the placental barrier.
Diseases like CHF, Uremia, Cirrhosis
In pathological states such as CHF, uremia, cirrhosis of the liver, etc.
can alter the volume of drug distribution of many drugs by altering the distribution of body water, the permeability of membranes, binding proteins, or the accumulation of metabolites that displace drugs from binding sites.
Question 8. Explain The Pattern Of Drug Excretion And the Factors Affecting It.
Answer:
Pattern Of Drug Excretion And Factors Affecting
Excretion is the passage out of a systemically absorbed drug.
Drug and their metabolites are excreted in:
- Urine: The kidney is the most important channel of excretion for most drugs.
- Feces: Unabsorbed fraction and the drug derived from the bile (relatively larger molecules)
Certain drugs are excreted directly in the colon, for Example. anthracene, purgatives, and heavy metals - Exhaled air: Gases and volatile liquids (general anesthesia) are eliminated by the lungs. Alveolar transfer of gas depends on its partial pressure in the blood.
- Saliva and sweat: These are of minor importance for drug excretion. Lithium, thiocyanates, rifampicin, and heavy metals are present in these secretions.
- Milk: Not important for the mother, but the sucking infant inadvertently receives the drug. More lipid soluble and less protein bound drugs cross better.
Renal Excretion
- A most important channel for drug excretion.
- The amount of drug or its metabolites ultimately present in urine is sum total of glomerular filtration, tubular reabsorption, and tubular secretion.
Glomerular Filtration
- Glomerular capillaries have pores larger than usual, and all non-protein bounded drug present in the glomerulus is filtered.
- The glomerular filtration of the drug depends on its plasma protein binding and renal blood flow.
- GFR declines progressively after the age of 50.
Tubular Reabsorption
- This depends on the lipid solubility and ionization of the drug at the existing urinary pH.
- Lipid-soluble drugs filtered at the glomerulus back differ in tubules but non-lipid soluble and highly ionized drugs are unable to do so.
- Changes in urinary pH affect tubular reabsorption of drugs that are partially ionized. Weak bases ionize more and are less reabsorbed in acidic urine.
- Weak acids ionize more and are less reabsorbed in alkaline urine.
Tubular Secretion
This is the active transport of organic acids and bases by two separate non-specific mechanisms which operate in proximal tubules.
- Organic acid transport: For penicillin, probenecid, uric acid, salicylates, sulfipyrazone, methotrexate, etc.
- Organic base transport: For thiazides, quinine, procainamide, choline, amiloride, etc.
- For drugs and their metabolite secretion into the tubular lumen predominates, whereas an endogenous substrate like uric acid is predominantly reabsorbed.
- Tubular transport mechanisms are not well developed at birth. As a result, the duration of action of many drugs, for Example. penicillin, aspirin, and cephalosporin are longer in neonates.
- These systems mature during infancy.
Question 9. Explain The Pharmacological Basis For The Ph Of Urine Is Altered In Poisoning Cases.
Answer:
The Pharmacological Basis For The Ph Of Urine
In cases of poisoning, the main aim of the treatment is to cause early excretion of poison from the body as soon as possible. The excretion of the drug depends on the pH of the urine.
Drugs are present in a lipid-soluble form in the same medium whereas in the opposite medium drugs get ionized and become water-soluble.
So in cases of poisoning by acidic drugs, excretion of the drug is enhanced by making urine basic.
This causes ionizing of the drug and it is quickly excreted in the urine.
Similarly in cases with basic drug poisoning urine is made acidic by ammonium chloride.
Question 10. Write In Short On Phase II Metabolism.
Answer:
Phase II Metabolism
Phase II metabolism is also known as the synthetic or conjugation phase:
- This Phase II metabolism is mostly inactive.
- Phase II reactions involve the conjugation of the drug or its phase I metabolite with an endogenous substrate, generally derived from carbohydrates or amino acids. To form a polar highly ionized organic acid, which is easily excreted in urine or bile.
- Conjugation reactions have high energy requirements.
- Glucuronide conjugation: This is the most important synthetic reaction carried out by a group of UDPglucuronosyl transferases. Compounds with a hydroxyl or carboxylic acid group are easily conjugated with glucuronic acid which is derived from glucose.
Examples are chloramphenicol, aspirin, etc. Glucuronidation increases the molecular weight of the drug which favors its excretion in bile. - Acetylation: Compounds having amino or hydrazine residues are conjugated with the help of acetyl coenzyme-A.
Example. sulfonamides, isoniazid, hydralazine. Multiple genes control the N-acetyl transferases, and the rate of acetylation shows genetic polymorphism. - Methylation: The amines and phenols can be methylated; methionine and cysteine act as methyl donors, for Example. adrenaline, histamine, nicotinic acid.
- Sulfate conjugation: The phenolic compounds and steroids are sulfated by sulfotransferases, for Example. chloramphenicol, methyldopa, adrenal, and sex steroids.
- Glycine conjugation: Salicylates and other drugs having carboxylic acid groups are conjugated with glycine, but this is not a major pathway of metabolism.
- Glutathione conjugation: Forming a mercapturic is normally a minor pathway. However, it serves to inactivate highly reactive quinone or epoxide intermediates formed during the metabolism of certain drugs, for Example. paracetamol.
When a large number of such intermediates are formed glutathione supply falls short then toxic adducts are formed with tissue constituents and lead to tissue damage. - Ribonucleoside/nucleotide synthesis: This pathway is important for the activation of many purine and pyrimidine antimetabolites used in cancer chemotherapy.
- Glucuronide conjugation: This is the most important synthetic reaction carried out by a group of UDPglucuronosyl transferases. Compounds with a hydroxyl or carboxylic acid group are easily conjugated with glucuronic acid which is derived from glucose.
Question 11. Define Pharmacokinetics. Explain The Factors Affecting
Bioavailability Of Drug.
Answer:
Pharmacokinetics
Pharmacokinetics is the quantitative study of drug movement in, through, and out of the body.
Factors Affecting The Bioavailability Of Drug
- Disintegration and dissolution of time: Oral drugs have to disintegrate to be absorbed and then dissolved in gastrointestinal fluids. Liquids are absorbed faster than solids. Water-soluble drugs like aspirin reduce bioavailability.
- Formulation: Inert substances used with drugs as diluents like starch has slow absorption and reduce bioavailability.
- Particle size: Small particles are more easily absorbed than bigger particles and there is better bioavailability.
- Lipid solubility: Lipid-soluble drugs are absorbed faster and they are more bioavailable.
- PH and ionization: Ionized drugs are poorly absorbed compared to unionized drugs. Acidic drugs remain unionized in an acidic medium and are rapidly absorbed, for Example. aspirin, barbiturates.
- Area and vascularity of the absorbing surface: The larger the surfaces are for absorption and more vascularity, there will be more absorption and bioavailability.
- Gastrointestinal motility: Faster gastric emptying time ensures passage of the drug to the intestine is fast and more absorption. Intestinal motility as in diarrhea reduces absorption and the amount of drug available is reduced.
- Presence of food: Delays gastric emptying and delays absorption.
- Metabolism: Some drugs are degraded in the gastrointestinal tract and bioavailability becomes zero.
- Diseases: Bioavailability is increased in patients with liver disorders due to a reduction in hepatic metabolism.
Question 12. Write A Short Note On Therapeutic Drug Monitoring.
Answer:
Therapeutic Drug Monitoring
Therapeutic drug monitoring is the process by which the dose of a drug is adjusted according to its plasma concentration.
Indications
- It is done for the drugs having a known correlation between serum level and toxicity.
- It is done for the drugs which have wide variation in pharmacokinetics both intra as well as inter-individual.
- It is done for those drugs whose effects cannot be easily measured.
- It is not done for the drugs which are activated in the body or produce active metabolites.
- It is done for those drugs whose toxicity may lead to hospitalization or irreversible organ damage and these adverse drug reactions are avoided by using therapeutic drug monitoring.
Therapeutic dose monitoring is not required when:
- Clinical and biochemical parameters are available to assess response.
- Drugs leading to tolerance, for Example. opioids.
- In drugs whose effects persist longer than the drug itself, for Example. omeprazole.
Examples Of Drugs Analysed By Therapeutic Drug Monitoring
- Aminoglycoside antibiotics (gentamicin)
- Antiepileptics (such as carbamazepine, phenytoin and valproic acid)
- Mood stabilizers, especially lithium citrate
- Antipsychotics (such as pimozide and clozapine).
Question 13. Write In Short Enzyme Induction.
Or
Write Short Note On Enzyme Induction.
Answer:
Enzyme Induction
Repeated administration of certain drugs leads to an increase in the synthesis of microsomal enzymes. This is known as enzyme induction.
Enzymatic induction is more prominent in the liver and also occurs in the lungs, placenta, and kidney.
1. Importance Of Enzyme Induction
- Enzyme induction can accelerate the metabolism of drugs and reduces the duration and intensity of drug action which causes therapeutic failure.
- Autoinduction can lead to the development of drug tolerance.
- Enzyme induction can cause drug toxicity.
- Prolonged phenytoin therapy may produce osteomalacia due to enhanced metabolism of vitamin D3.
- Enzyme inducers may precipitate porphyria because of the overproduction of porphobilinogen.
- Enzyme induction can be beneficial too as phenobarbitone induces glucuronyl transferase enzyme hence bilirubin is conjugated and jaundice is resolved.
2. Inducers Of Cyt-P450 Complex
Drugs that increase the production of Cytochrome-P450 enzymes:
- Anti-convulsants: Phenobarbitol, Phenytoin, and Carbamazepine induce CYT-P450-3A4 enzyme.
- Phenobarbitol and Phenytoin also induce the CYT-P450-2Bl enzyme.
- Polycyclic Aromatics (PAH): induce CYT-P450-1Al enzyme.
- Glucocorticoids induce CYT-P450-3A4 enzyme.
- Chronic alcohol, isoniazid induces CYT-P450-2E1 enzyme.
This is important as this drug activates some carcinogens
such as nitrosamines. - Chronic alcoholics have up-regulated many of their CYT-P450 enzymes.
3. Uses Of Enzyme Induction
- In congenital non-hemolytic jaundice
- In Cushing’s syndrome
- In chronic poisonings
- In liver disease.
Question 14. Write A Short Note On First Pass Metabolism.
Answer:
First Pass Metabolism
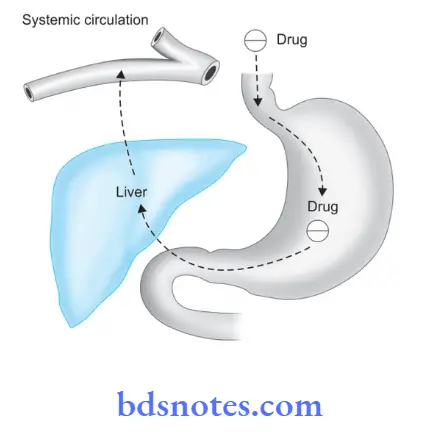
It is also known as pre-systematic metabolism.
- First-pass metabolism refers to the metabolism of the drug during its first passage from the site of absorption into the systemic circulation.
- All orally administered drugs have to pass via the gut wall, portal vein, and liver to enter the systemic circulation.
Some drugs during this process of passage get metabolized and are removed or inactivated before they reach the systemic circulation, this process is known as first-pass metabolism. - First-pass metabolism results in decreased bioavailability as well as decreased therapeutic effect of the drug, for Example. propranolol and nitroglycerine.
Consequences Of High First-Pass Metabolism
- Drugs that undergo extensive first-pass metabolism should be given parenterally.
- Drug dosage which is required for oral administration is higher as compared to other systemic routes.
Question 15. Write A Short Note On The Volume Of Distribution.
Answer:
Volume Of Distribution
The volume of distribution is defined as the apparent volume of plasma required to contain the administered dose if that dose was evenly distributed at the concentration measured in plasma.
If more amount of drugs enters the tissues, it has a higher volume of distribution and vice-versa. It is denoted by Vd.
- The volume of distribution depends on factors such as lipid solubility and plasma protein binding.
- Lipid-soluble drugs are more likely to cross blood vessel walls and have a high volume of distribution.
- If the drug is highly bound to plasma proteins it will behave like a large molecule and is more likely to stay in plasma.
So, therefore, the amount of drug supplied to the tissues is less and it results in a reduced volume of distribution. - The volume of distribution is calculated by dividing plasma concentration attained (Co) by the dose of a drug administered IV.
Clinical Significance Of Volume Of Distribution
- It is the measure of the distribution of drugs. If Vd is more, it means there are more amount of drugs in the tissues and fewer in the plasma.
So initially high dose is administered to attain the therapeutic plasma concentration for drugs having high Vd than those having low Vd.
This high dose is known as the loading dose. So Vd is the main determinant of the loading dose. - Half-life is directly proportional to Vd. So drugs having high Vd are long-acting.
- Drugs with a high volume of distribution cannot be easily removed by dialysis as dialysis removes drugs only from plasma.
Question 16. Write A Short Note On The Fist And Zero Order Of Kinetics Of Drug Elimination.
Answer:
First Order Of Kinetics Of Drug Elimination
- It is also known as exponential kinetics.
- In this drug is eliminated in a fixed proportion (%) of the amount present in the body, in a unit period. The amount excreted therefore will progressively decrease.
- In the first order of kinetics, clearance is constant, therefore.
- A constant fraction of the drug is eliminated at a constant interval of time.
- The rate of drug elimination is directly proportional to its plasma concentration.
- After a single dose, about 97% of the drug gets eliminated after five (5) half-lives (t½).
- If a fixed dose of the drug is administrated at every (t½), at least 4 to 5 (t½) would be needed for the achievement of its steady-state plasma
concentration in the body. - The majority of drug obeys first-order kinetics of elimination.
Zero Order Of Kinetics Of Drug Elimination
- It is also known as linear kinetics.
- In this, a fixed or constant amount of drug is eliminated in a given period of time irrespective of its amount or the concentration present at that time.
In this case rate of elimination is independent of the concentration of the drug in plasma.- A plot of drug concentration vs time is linear
- t½ of drugs following zero order kinetics of elimination is never constant.
- Hardly few drugs follow zero-order kinetics in the true sense.
Question 17. Write A Short Note On The Acetylation Of Drugs.
Answer:
Acetylation Of Drugs
Compounds having amino or hydrazine residues are conjugated with the help of acetyl coenzyme-A, for Example. sulfonamides, isoniazid, hydralazine.
- Multiple genes control the N-acetyl transferases, and the rate of acetylation shows genetic polymorphism.
- Acetylation of the drug is a non-microsomal conjugation.
- Slow acetylation is due to an autosomal recessive gene which is associated with a smaller amount of hepatic-N-acetylase.
- Fast acetylation is due to an autosomal dominant gene that is associated with a large amount of hepaticN-acetylase.
- Slow acetylators are more likely to accumulate the drug and lead to experience adverse reactions.

Leave a Reply