Inflammation
Question 1. Define inflammation and describe cardinal signs of inflammation. Describe vascular events of inflammation.
Or
Describe in brief cardinal signs and vascular changes in inflammation.
Or
Discuss the pathogenesis of vascular phenomenon in acute inflammation.
Or
Describe the vascular and cellular response of acute inflammation.
Or
Write a short note on vascular events of acute inflammation.
Or
Enumerate cardinal signs of inflammation and describe in brief vascular events of acute inflammation.
Or
Describe vascular events in acute inflammation.
Answer:
Definition: Inflammation is defined as the local response of living mammalian tissues to injury due to any agent. It is a body defense reaction in order to eliminate or limit the spread of injurious agents as well as to remove the consequent necrosed cells and tissues.
Cardinal Signs of Inflammation
The four cardinal signs of inflammation proposed by Celsus in the first century AD are as follows:
- Rubor or redness
- Tumor or swelling
- Calor or heat
- Dolor or pain.
Read And Learn More: Pathology Question And Answers
To these above four signs, the fifth sign is added by Virchow, i.e. function laksa or loss of function.
Vascular Events of Inflammation
Alteration in the microvasculature (arterioles, capillaries and venules). These alterations include:
- Hemodynamic changes
- Changes in vascular permeability.
Hemodynamic Changes
- Transient vasoconstriction: Irrespective of the type of cell injury, the immediate vascular response is of transient vasoconstriction of arterioles. With the mild form of injury, the blood flow may be re-established in 3 to 5 seconds while with more severe injury the vasoconstriction may last for about 5 minutes.
- Persistent progressive vasodilatation: Next follows persistent progressive vasodilatation which involves mainly the arterioles, but to a lesser extent affects other components of the microcirculation like venules and capillaries. This change is obvious within half an hour of injury. Vasodilatation results in increased blood volume in the microvascular bed of the area, which is responsible for redness and warmth at the site of acute inflammation.
- Local hydrostatic pressure: Progressive vasodilatation, in turn, may elevate the local hydrostatic pressure resulting in the transudation of fluid into the extracellular space. This is responsible for swelling at the local site of acute inflammation.
- Slowing or stasis: Slowing or stasis of microcirculation follows which causes an increased concentration of red cells, and thus, raised blood viscosity.
- Leucocyte margination: Stasis or slowing is followed by leucocytic margination or peripheral orientation of leucocytes (mainly neutrophils) along the vascular endothelium. The leucocytes stick to the vascular endothelium briefly, and then move and migrate through the gaps between the endothelial cells into the extravascular space. This process is known as emigration.
Patterns of Increased Vascular Permeability:
Increased vascular permeability in acute inflammation by which normally non-permeable endothelial layer of microvasculature becomes leaky can have following patterns and mechanisms which may be acting singly or more often in combination:
- Contraction of endothelial cells: This is the most common mechanism of increased leakiness that affcts venules exclusively while capillaries and arterioles remain unaffected. The endothelial cells develop temporary gaps between them due to their contraction resulting in vascular leakiness. It is mediated by the release of histamine, bradykinin and other chemical mediators. The response begins immediately after injury, is usually reversible, and is for a short duration (15-20 minutes). An example of such an immediate transient response is a mild thermal injury of the skin of forearm.
- Contraction or mild endothelial damage: In this mechanism, there is a structural reorganization of the cytoskeleton of endothelial cells that causes reversible retraction at the intercellular junctions or mild form of endothelial damage. This change affects venules and capillaries and is mediated by cytokines such as interleukin-1 (IL-1) and tumor necrosis factor (TNF)-a. The onset of response occurs after delay of 4-6 hours following injury and lasts for several hours to days. A classic example of delayed and prolonged leakage is the appearance of sunburns mediated by ultraviolet radiation.
- Direct injury to endothelial cells: Direct injury to the endothelium causes cell necrosis and the appearance of physical gaps at the sites of detached endothelial cells. Process of thrombosis involving platelets and firin is initiated at the site of damaged endothelial cells. The change affcts all levels of microvasculature (venules, capillaries and = arterioles). The increased permeability may either appear immediately after injury and last for several hours or days (immediately sustained leakage), or may occur after a delay of 2-12 hours and last for hours or days (delayed prolonged leakage). Examples of immediate sustained leakage are severe bacterial infections while delayed prolonged leakage may occur following moderate thermal injury and radiation injury.
- Leucocytemediated endothelial injury: Adherence of leucocytes to the endothelium at the site of inflammation may result in activation of leucocytes. The activated leucocytes release proteolytic enzymes and toxic oxygen species which may cause endothelial injury and increased vascular leakiness. This form of increased vascular leakiness affcts mostly venules and is a late response. The examples are seen in sites where leucocytes adhere to the vascular endothelium, For Example. in pulmonary venules and capillaries.
- Leakiness in neovascularization: In addition, the newly formed capillaries under the influence of vascular endothelial growth factor (VEGF) during the process of repair and in tumors are excessively leaky.
Question 2. Give a brief account of cellular events in acute inflammation.
Or
Describe exudation of leucocytes inacute inflammation.
Answer:
The cellular phase of inflammation consists of two processes:
- Exudation of leucocytes.
- Phagocytosis.
Cellular events in acute inflammation Exudation of Leucocytes:
The escape of leucocytes from the lumen of the microvasculature to the interstitial tissue is the most important feature of inflammatory response. In acute inflammation, polymorphonuclear neutrophils (PMNs) comprise the first line of body defense, followed later by monocytes and macrophages. The changes leading to the migration of leucocytes are as follows:
1. Changes in the formed elements of blood: In the early stage of inflammation, the rate of flw of blood is increased due to vasodilatation. But subsequently, there is slowing or stasis of the bloodstream.
With stasis, changes in the normal axial fl0w of blood in the microcirculation take place. The normal axial flow consists of central stream of cells comprised by leucocytes and RBCs and a peripheral cell-free layer of plasma close to vessel wall.
Due to slowing and stasis, the central stream of cells widens and the peripheral plasma zone becomes narrower because of loss of plasma by exudation.
This phenomenon is known as margination. As a result of this redistribution, neutrophils of the cen- tral column come close to the vessel wall; this is known as pavements.
2. Rolling and adhesion: Peripherally marginated and pave mounted neutrophils slowly roll over the endothelial cells lining the vessel wall (rolling phase).
This is followed by the transient bond between the leucocytes and endothelial cells becoming firmer (adhesion phase). The following cell adhesion molecules (CAMs) bring about rolling and adhesion phases:
- Selectins: These are a group of cell adhesion molecules expressed on the surface of activated endothelial cells and are structurally composed of lectins or lectin-like protein molecules the most important of which is s-Lewis X molecule. Their role is to recognize and bind to glycoproteins and glycolipids on the cell surface of neutrophils. There are 3 types of selectins viz P-selectin, E-selectin and L-selectin.
- Integrins: These are a family of endothelial cell sur- face proteins having alpha (or CD11) and beta (CD18) subunits, which are activated during the process of loose and transient adhesions between endothelial cells and leucocytes. At the same time the receptors for integrins on the neutrophils are also stimulated. This process brings about fim adhesion between leucocyte and endothelium.
- Immunoglobulin gene superfamily adhesion molecules: This group consists of a variety of immuno- globulin molecules present on most cells of the body. These take part in cell-to-cell contact through various other cell adhesion molecules and cytokines. They have a major role in the recognition and binding of immunocompetent cells as under Intercellular adhesion molecule-l and vascular cell adhesion molecule-1 allow a tighter adhesion and stabilize the interaction between leucocytes and endothelial cells.
Platelet-endothelial cell adhesion molecule-1 or CD31 is involved in leucocyte migration from the endothelial surface.
3. Emigration: After sticking of neutrophils to the endothelium, the former move along the endothelial surface till a suitable site between the endothelial cells is found where the neutrophils throw out cytoplasmic pseudopods.
Subsequently, the neutrophils lodged between the endothelial cells and basement membrane cross the basement membrane by damaging it locally with secreted colla geniuses and escape out into the extravascular space; this is known as emigration.
The damaged basement membrane is repaired almost immediately. Neutrophils are the dominant cells in acute inflammatory exudate in the first 24 hours, and monocyte-macrophages appear in the next 24-48 hours.
However, neutrophils are short-lived (24-48 hours) while monocyte-macrophages survive much longer. Simultaneous to the emigration of leucocytes, and escape of red cells through gaps between the endothelial cells, diapedesis takes place.
It is a passive phenomenon of RBCs being forced out either by raised hydrostatic pressure or may escape through the endothelial defects left after the emigration of leucocytes. Diapedesis gives her- a tragic appearance to the inflammatory exudate.
4. Chemotaxis: The transmigration of leucocytes after crossing several barriers to reach the interstitial tissues is a chemotactic factor-mediated process called chemotaxis. The following agents act as potent chemotactic substances for neutrophils:
- Leukotriene B4 (LT-B4), a product of the lipooxygenase pathway of arachidonic acid metabolites
- Components of complement system (C5a and C3a in particular)
- Cytokines (interleukins, in particular, IL-8)
- Soluble bacterial products (such as formylated pep-tides).

Cellular events in acute inflammation Phagocytosis:
Phagocytosis is defined as the process of engulfment of solid particulate material by the cells (cell—eating). The cells perform- ing this function are called phagocytes. There are 2 main types of phagocytic cells:
- Polymorphonuclear neutrophils (PMNs) which appear early in acute inflammatory response, are sometimes called as macrophages.
- Circulating monocytes and fixed tissue mononuclear phagocytes, commonly called as macrophages. Neutrophils and macrophages on reaching the tissue spaces produce several proteolytic enzymes—lysozyme, protease, collagenase, elastase, lipase, proteinase, gelatinase and acid hydrolases. These enzymes degrade collagen and extracellular matrix.
Phagocytosis of the microbe by polymorphs and macrophages involves the following 3 steps:
- Recognition and attachment
- Engulfment
- Killing and degradation.
Recognition and Attachment
Phagocytosis is initiated by the expression of cell surface receptors on macrophages which recognize micro organisms mannose receptor and scavenger receptor.
The process of phagocytosis is further enhanced when the microorganisms are coated with specific proteins, opsonins, from the serum and the process is called opsonization.
Opsonins establish a bond between bacteria and the cell membrane of phagocytic cell. The main opsonins present in the serum and their corresponding receptors on the surface of phagocytic cells (PMNs or macrophages) are as under:
- IgG opsonin is the Fc fragment of immunoglobulin G; it is the naturally occurring antibody in the serum that coats the bacteria while the PMNs possess receptors for the same.
- C3b opsonin is the fragment generated by the activation of the complement pathway. It is strongly chemotactic for attracting PMNs to bacteria.
- Lectins are carbohydrate-binding proteins in the plasma which bind to bacterial cell wall.
Engulfment
The opsonized particle or microbe bound to the surface of the phagocyte is ready to be engulfed.
This is accomplished by the formation of cytoplasmic pseudopods around the particle due to activation of actin filaments beneath cell wall, enveloping it in a phagocytic vacuole.
Eventually, plasma membrane enclosing the particle breaks from the cell surface so that membrane—lined phagocytic vacuole or phagosome becomes internalized in the cell and lies free in the cell cytoplasm.
The phagosome fuses with one or more lysosomes of the cell and form a bigger vacuole called a phagolysosome.
Killing and Degradation
Next is the stage of killing and degradation of microorganisms to dispose it of which is the major function of phagocytes as scavenger cells. The microorganisms after being killed by antibacterial substances are degraded by hydrolytic enzymes.
However, this mechanism fails to kill and degrade some of bacteria like tubercle bacilli. The following are the mechanisms involved in the disposal of microorganisms:
1. Intracellular Mechanisms:
Intracellular metabolic pathways are involved in killing microbes, more commonly by oxidative mechanisms and less often by non-oxidative pathways.
- Oxidative bactericidal mechanism by oxygen free radicals An important mechanism of microbicidal killing is by oxidative damage by the production of reactive oxygen metabolites. A phase of increased oxygen consumption by activated phagocytic leucocytes requires the essential presence of NADPH oxidase. NADPH oxidase present in the cell membrane of phagosome reduces oxygen to superoxide ion. This type of bactericidal activity is carried out either via enzyme myeloperoxidase (MPO) present in the azurophilic granules of neutrophils and monocytes, or independent of enzyme MPO, as under:
- MPO-dependent killing: In this mechanism, the enzyme MPO acts on H2O2 in the presence of halides (chloride, iodide or bromide) to form hypohalous acid (HOCl, HOl, HOBr). This is called H2O2 – MPO halide system and is more potent antibacterial system in polymorphs than H2O2 alone:
- MPO independent killing: Mature macrophages lack the enzyme MPO and they carry out the bactericidal activity by producing OH- ions and superoxide singlet oxygen (O’) from H2O2 in the presence of O’ 2 or in the presence of Fe++ (Fenton reaction): Reactive oxygen metabolites are particularly useful in eliminating microbial organisms that grow within phagocytes For Example. Mycobacterium tuberculosis, Histoplasma capsulatum.
- The oxidative bactericidal mechanism by lysosomal granules: In this mechanism, the preformed granule-stored products of neutrophils and macrophages are discharged or secreted into the phagosome and the extracellular environment. While the role of MPO is already highlighted above, other substances liberated by the degranulation of macrophages and neutrophils are protease, trypsinase, phospholipase, and alkaline phosphatase. Progressive degranulation of neutrophils and macrophages along with oxygen free radicals degrades proteins, i.e. induces proteolysis.

- Non-oxidative bactericidal mechanism: Some agents released from the granules of phagocytic cells do not require oxygen for bactericidal activity. These include the following:
- Granules: Some of the liberated lysosomal granules do not cause killing by oxidative damage but cause lysis of microbe within the phagosome. These are lysosomal hydrolases, permeability-increasing factors, cationic proteins (defensins), lipases, proteases, and DNAases.
- Nitric oxide: Nitric oxide is reactive free radicals similar to oxygen free radicals which are formed by nitric oxide synthase. It is produced by endothelial cells as well as by activated macrophages. Nitric oxide is another potential mechanism of microbial killing.
2. Extracellular Mechanisms:
The following mechanisms explain the bactericidal activity at the extracellular level:
- Granules: Degranulation of macrophages and neutrophils explained above continues to exert its effects of proteolysis outside the cells as well.
- Immune mechanisms: Immune-mediated lysis of microbes takes place outside the cells by mechanisms of cytolysis, antibody-mediated lysis, and cell-mediated cytotoxicity.
Question 3. Describe characteristic features of acute inflammation.
Answer:
Characteristic Features of Acute Inflammation
- The main characteristic features of acute inflammation are:
- Accumulation of fluid and plasma at the affected side.
- Intravascular activation of platelets.
- PMN as inflammatory cells.
Question 4. Describe the chemical mediators of acute inflammation.
Or
Describe in brief the chemical mediators of inflammation.
Or
Write a short note on chemical mediators of inflammation.
Or
Write in detail the chemical mediators of inflammation and their role in inflammation.
Or
Mention the names of chemical mediators of acute inflammation and describe the functions of any two of them.
Answer:
Chemical Mediators of Acute Inflammation
There are many biochemical substances in the body which become active in the area of inflammation. These substances mostly increase vascular permeability or the production of pain. They are broadly classified into two groups:
- Mediators released by cell
- Mediators are released by plasma.
Cell-Derived Mediators
- Vasoactive amines, i.e. histamine, 5-hydroxytryptamine, neuropeptides
- Arachidonic acid metabolites (eicosanoids)
- Metabolites via cyclo-oxygenase pathway, i.e. prostate- glands, thromboxane A2, prostacyclin, resolvins
- Metabolites via the lipo-oxygenase pathway, i.e. 5-HETE, leukotrienes, lipoxins
- Lysosomal components from PMNs, macrophages
- Platelet-activating factor
- Cytokines, i.e. IL-1, IL-6, IL-8, IL-12, IL-17, TNF-a, TNF-β, lFN-?, chemokines
- Free radicals, i.e. oxygen metabolites, nitric oxide
Plasma ProteinDerived Mediators (Plasma Proteases) Products of:
- The kinin system
- The clotting system
- The fibrinolytic system
- The complement system.
1. Vasoactive Amines
- Histamine: It is stored in the granules of mast cells, basophils and platelets. Histamine is released from these cells by various agents as under:
- Stimuli or substances inducing acute inflammation, e.g. heat, cold, irradiation, trauma, irritant chemicals, immunologic reactions, etc.
- Anaphylatoxins like fragments of complement C3a, and C5a, increase vascular permeability and cause edema in tissues.
- Histamine-releasing factors from neutrophils, monocytes, and platelets.
- Interleukins.
The main actions of histamine are vasodilatation, increased vascular permeability, itching and pain.
- 5-Hydroxytryptamine (5HT or serotonin): It is present in tissues like chromaffin cells of GIT, spleen, nervous tissue, mast cells and platelets. The actions of 5-HT are similar to histamine but it is a less potent mediator of increased vascular permeability and vasodilatation than histamine.
- Neuropeptides: Another class of vasoactive amines is tach-kinin neuropeptides such as substance P, neurokinin A, vasoactive intestinal polypeptide and somatostatin. These small peptides are produced in central and peripheral nervous systems. The major proinflammatory action of these neuropeptides are:
- Increased vascular permeability
- Transmission of pain stimuli
- Mast cell degranulation
2. Arachidonic Acid Metabolites (Eicosanoids):
Arachidonic acid metabolites or eicosanoids are the most potent mediators of inflammation, much more than oxygen free radicals. Arachidonic acid is released from the cell membrane by phospholipases.
It is then activated to form arachidonic acid metabolites or eicosanoids by one of the following 2 pathways: via cyclo-oxygenase pathway or via lipo-oxygenase pathway:
- Metabolites via cyclo-oxygenase pathway: Prostaglan- dins, thromboxane A2, prostacyclin
-
- Prostaglandins (PGD2, PGE2, and PGF2-α): PGD2 and PGE2 act on blood vessels and cause increased venular permeability, vasodilatation, and bronchodilatation and inhibit inflammatory cell function. PGF2-a induces vasodilatation and bronchoconstriction.
- Thromboxane A2 (TXA2): Platelets contain the enzyme thromboxane synthetase and hence the metabolite, thromboxane A2, formed is active in platelet aggregation, vasoconstrictor and bronchoconstrictor.
- Prostacyclin (PGI2): PGI2 induces vasodilatation, and bronchodilatation and inhibits platelet aggregation.
- Resolvins are another derivative of the COX pathway which act by inhibiting the production of proinflammatory cytokines. Thus, resolvins are actually helpful drugs such as aspirin which act by inhibiting COX activity and stimulating the production of resolvins.
- Metabolites via lipooxygenase pathway: 5-HETE, leukotrienes, lipoxins: The enzyme, lipooxygenase, a predominant enzyme in neutrophils, acts on activated arachidonic acid to form hydroperoxy eicosatetraenoic acid which on further peroxidation forms following two metabolites:
-
- 5-HETE: It is an intermediate product, is a potent chemotactic agent for neutrophils.
- Leukotrienes (LT): They are so named as they were fist isolated from leucocytes. Firstly, unstable leukotriene A4 (LTA4) is formed which is acted upon by enzymes to form LTB4 while LTC4, LTD4 and LTE4 have common actions by causing smooth muscle contraction and thereby inducing vasoconstriction, bronchoconstriction and increased vascular permeability.
- Lipoxins: They act to regulate and counterbalance the actions of leukotrienes. Lipooxygenase-12 present in platelets acts on LTA4, derived from neutrophils and forms LXA4 and LXB4.
3. Lysosomal Components:
The inflammatory cells neutrophils and monocytes contain lysosomal granules which on release elaborate a variety of mediators of inflammation. These are as under:
- Granules of neutrophils: Neutrophils have 3 types of granules: primary or azurophil, secondary or specific, and tertiary.
Primary or azurophil granules are large azurophil granules that contain functionally active enzymes. These are myeloperoxidase, acid hydrolases, acid phosphatase, lysozyme, defensin (cationic protein), phospholipase, cathepsin G, elastase, and protease.- Secondary or specific granules contain alkaline phosphatase, lactoferrin, gelatinase, collagenase, lysozyme, vitamin-B12 binding proteins, and plasminogen activator.
- Tertiary granules or C particles contain gelatinase and acid hydrolases.
- Myeloperoxidase causes oxidative lysis by the generation of oxygen free radicals, acid hydrolases act within the cell to cause the destruction of bacteria in phagolysosome while proteases attack on the extracellular constituents such as basement membrane, collagen, elastin, cartilage, etc.
- Granules of monocytes and tissue macrophages: These cells on degranulation also release mediators of inflammation like acid proteases, collagenase, elastase and plasminogen activators. However, they are more active in chronic inflammation than acting as mediators of acute inflammation.
4. Platelet Activating Factor:
It is released from IgE-sensitised basophils or mast cells, other leucocytes, endothelium and platelets. Apart from its action on platelet aggregation and release reaction, the actions of platelet-activating factor as a mediator of inflammation are:
- Increased vascular permeability
- Vasodilatation in low concentration and vasoconstriction otherwise
- Bronchoconstriction
- Adhesion of leucocytes to the endothelium
- Chemotaxis.
5. Cytokines:
Cytokines are polypeptide substances produced by activated lymphocytes and activated monocytes. Major cytokines and their role in inflammation are:
1. Interleukins (IL1, IL6, IL8, IL12, IL17): IL-l and IL-6 are active in mediating acute inflammation, IL-12 and IL-17 play a potent role in chronic inflammation. IL-8 is a chemokine for acute inflammatory cells:
- IL-1 is elaborated by several body cells—monocytes and macrophages, B lymphocytes, fibroblasts, endothelial, and some epithelial cells. Similarly, it can target all body cells. Its major actions are:
-
- Expression of adhesion molecules
- Emigration of neutrophils and macrophages
- Role in fever and shock
- Hepatic production of acute phase protein.
- IL-6 is similar in its sources and target cells of action. Its major role is:
- Hepatic production of acute phase protein
- Differentiation and growth of T and B cells.
- IL-8 is a chemokine and its major actions are:
- Induces migration of neutrophils, macrophages, and T cells
- Stimulates release of histamine from basophils
- Stimulates angiogenesis.
- IL-12 is synthesized by macrophages, dendritic cells, and neutrophils while it targets T cells and NK cells. Its actions in chronic inflammation are as under:
- Induces the formation of T helper cells and killer cells
- Promotes cytolytic activity
- Increases production of IFN-?
- Decreases production of IL-17.
- IL-17 is formed by CD4+T cells while it targets fibroblasts, endothelial cells, and epithelial cells. Its action in chronic inflammation are:
- Increased secretion of other cytokines
- Migration of neutrophils and monocytes.
2. Tumor necrosis factor (TNFa and β): TNF-a is a mediator of acute inflammation while TNF-β is involved in cellular cytotoxicity and in the development of spleen and lymph nodes. Major actions of TNF-a are:
- Hepatic production of acute phase proteins
- Systemic features (fever, shock, anorexia)
- Expression of endothelial adhesion molecules
- Enhanced leucocyte cytotoxicity
- Induction of pro-inflammatory cytokines.
3. Interferon (IFN)?: It is produced by T cells and NK cells and may act on all body cells. It acts as a mediator of acute inflammation as under:
- Activation of macrophages and NK cells
- Stimulates secretion of immunoglobulins by B cells
- Role in the differentiation of T helper cells.
6. Free Radicals: Oxygen Metabolites and Nitric Oxide
Free radicals act as potent mediators of inflammation:
- Oxygen-derived metabolites are released from activated neutrophils and macrophages and include superoxide oxygen, H2O2, OH- and toxic NO products. These oxygen-derived free radicals have the following actions in inflammation:
- Endothelial cell damage and thereby increased vascular permeability.
- Activation of protease and inactivation of antiprotease causing tissue matrix damage.
- Damage to other cells.
- Nitric oxide (NO): Nitrous oxide plays the following roles in mediating inflammation
- Vasodilatation
- Anti-platelet activating agent
- Possibly microbicidal action.
Chemical Mediators of Acute Inflammation Plasma Derived Mediators
There include various products derived from the activation and interaction of interlinked systems, kinin, clotting, fibrinolytic, and complement.
- The Kinin system: This system on activation by factor XII generates bradykinin. Bradykinin acts in the early stage of inflammation.
- Smooth muscle contraction
- Vasodilatation
- Increase vascular permeability
- Pain
- The clotting system: The action of fibrin peptides in inflammation are:
- Increase permeability
- Chemotaxis for leukocyte
- Anticoagulant activity.
- The fibrinolytic system: This system on activation by a plasminogen activator generates plasmin.
- The action of plasmin in inflammation is:
- Activation of factor XII to form a pro-kallikrein activator that stimulates the kinin system to generate bradykinin.
- Splits of complement C3 to form C3a which is a permeability factor.
- Degrades the firin to form firin split products which increase vascular permeability and are chemotactic of leucocytes.
- The action of plasmin in inflammation is:
- The complement system: The complement system on activation yields anaphylatoxins, C3a, C4a, C5a and membrane attack complex.
- The action of anaphylatoxins is as follows:
- Release histamine from mast cells and basophils
- Increase vascular permeability causing edema.
- C3 augments phagocytosis.
- C5a is chemotactic for leucocytes.
- The action of anaphylatoxins is as follows:
Question 5. Discuss common chronic inflammatory conditions in relation to dental pathology.
Answer:
Chronic inflammation and degeneration of supporting tissues of teeth resulting in teeth loss is a common condition.
- Inflammatory periodontal diseases affect adults more commonly.
- Pregnancy, puberty and use of drugs like Dilantin are also associated with periodontal disease more often.
- The disease begins as chronic marginal gingivitis, secondary to bacterial plaques around the teeth such as due to calculus on the tooth surface, impacted food, uncontrolled diabetes, tooth decay and ill-fitting dental appliances.
- The gingival sulcus acts as a convenient site for the lodgment of food debris and bacterial plaque leading to the formation of periodontal pockets from which purulent discharge can be expressed by digital pressure.
- Pathological Features: Chronic marginal gingivitis is characterized by heavy chronic inflammatory cell infiltrate, destruction of collagen and epithelial hyperplasia so as to line the pocket. Untreated chronic marginal gingivitis slowly progresses to chronic periodontitis or pyorrhea.
Question 6. Discuss in brief granulomatous inflammation.
Or
Write a short note on granuloma.
Answer:
Granulomatous Inflammation
Granulomatous inflammation is a distinctive pattern of chronic inflammatory reaction characterized by the presence of granulomas.
Granuloma is defined as a circumscribed tiny lesion, about 1 mm in diameter composed predominantly of a collection of modified macrophages called epithelioid cells and rimmed at the periphery by lymphoid cells.
Evolution of Granuloma
Constituents of Granuloma
- Epithelioid cells
- Lymphoid cells
- Giant cells
- Necrosis
- Fibrosis.
Examples of Granulomatous Inflammation
- Tuberculosis
- Syphilis
- Leprosy
- Fungal infection.
Fate of Granuloma

Question 7. Write a short note on primary pulmonary tuberculosis.
Or
Write notes on Ghon’s complex.
Answer:
The infection in an individual who has not been previously infected or immunized is called primary tuberculosis or Ghon’s complex or childhood tuberculosis.
Ghon’s complex is the lesion produced in the tissue portal of entry with foci in draining lymphatic vessels and lymph nodes.
- The most commonly involved tissues for the primary complex are the lung and hilar lymph nodes. Other sites are the intestine, skin and oropharynx
- The primary complex or Ghon’s complex in the lungs consists of three components.
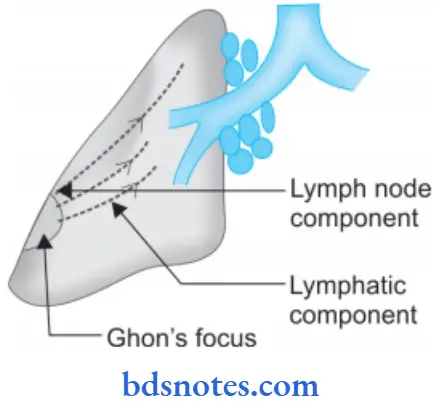
Ghon’s complex Pulmonary Component
The lesion in the lung is the primary focus or Ghon’s focus. It is 1-2 mm solitary area of tuberculous pneumonia located peripherally under a patch of pleurisy in any part of lung but more often in subpleural focus in upper part of lower lobe.
Microscopically: The lung lesion consists of tuberculosis granuloma with caseation necrosis.
Ghon’s complex Lymphatic Vessel Component:
The lymphatics draining the lung lesion consist of phagocytes containing bacilli and may develop beaded, miliary tubercles along the path of hilar lymph nodes.
Ghon’s complex Lymph Node Component:
This consists of enlarged hilar and tracheobronchial lymph nodes in the area drained. The affected lymph nodes are matted and show caseation necrosis.
Ghon’s complex Microscopically
Microscopically lesions of primary tuberculosis consist of the following features, i.e.
- Tuberculous granulomas with peripheral fibrosis
- Extensive caseation necrosis in the center of granulomas
- Old lesions have fibrosis and calcification.
Ghon’s Complex Fate of Pulmonary Tuberculosis
The primary complex may have one of the following sequelae:
- The lesion of primary tuberculosis of lung commonly do not progress but instead heal by fibrosis and in time undergoes calcification and even ossification.
- In some cases, the caseous material is disseminated through bronchi to other parts of the same lung or the opposite lung. This is called “progressive primary tuberculosis.”
- At times, bacilli may enter into circulation through erosion in a blood vessel and spread to various tissue and organiser: This is called as “primary miliary tuberculosis.”
- In some cases, the heard lesion of primary tuberculosis may get reactivated causing “secondary progressive tuberculosis”.
Question 8. Write about laboratory investigation done in case suffering from pulmonary tuberculosis.
Or
Write a short note on laboratory diagnosis of tuberculosis.
Or
Write a short note on the primary complex.
Answer:
The laboratory diagnosis of tuberculosis is done by:
Laboratory diagnosis of tuberculosis Diagnosis
1. Demonstration of AFB on microscopic examination of a diagnostic specimen (sputum or tissue): Smears or tissue slides stained by Ziehl-Neelsen stain are examined for acid-fast bacilli.
This method has a relatively low sensitivity in confirmed cases of pulmonary tuberculosis. Auramine-rhodamine staining and fluorescence microscopy can improve sensitivity to a certain extent.
Three sputum specimens preferably collected early in the morning should be submitted to the laboratory for AFB smear and mycobacterial culture.
2. Culture: Besides sputum and tissue other specimens which can be used for culture are body cavity fluids, urine or gastric lavage fluid.
Specimens may be inoculated onto egg or agar-based medium, For Example. Lowenstein- Jensen or Middle- brook 7H10 media and incubated at 37°C.
M. tuberculosis grows slowly (4-8 weeks). A presumptive diagnosis can be made based on colony pigmentation and morphology; however, biochemical tests are must for species recognition.
3. Molecular typing: M. tuberculosis is isolated and species identification is done by molecular methods or high-pressure liquid chromatography of mycolic acids (reducing the time required for a confirmation to 2-3 weeks). Polymerase chain reaction, i.e. PCR is the conformational method.
4. Tuberculin sensitivity test: It is based on the principle that M. tuberculosis in a concentrated liquid culture medium i.e. purified protein extract can elicit a skin reaction when injected subcutaneously into patients with tuberculosis.
A person is given the tuberculin and asked to return within 48-72 hours to have a trained health care worker to look for a reaction on the arm (swelling, induration and erythema) and measure its size.
Redness by itself is not considered part of the reaction. The lack of mycobacterial species specificity, the subjectivity of interpretation and batch-to-batch variations limit the usefulness of protein purified derivative.
5. In vitro assays that measure T cell release of IFN? in response to stimulation with the highly tuberculosisspecifi antigens ESAT6 and CPP10:
These are commercially available assays (Interferon-? release assay or IGRA) IGRAs are more specific than the tuberculin sensitivity test as a result of less cross-reactivity due to BCG vaccination and sensitization by non-tuberculous mycobacteria.
IGRAs also appear to be at least as sensitive as the tuberculin-sensitive test for active tuberculosis.
6. Complete hemogram: It shows lymphocytosis and raised ESR.
7. Fine needle aspiration cytology: This is done in the enlarged peripheral lymph node and is an easy way for confirm of the diagnosis
8. Immunohistochemistry: Immunohistochemical stain with anti-MBP 64 antibody stain can be used to demonstrate the organism.

Question 9. Write a short note on tuberculin reaction.
Answer:
This test is done by intradermal injection of 0.1 mL of tuberculin protein, a purified protein derivative.
- Delayed type of hypersensitivity develop in individuals who are having or have been previously infected with tuberculous infection which is identified as an indurated area of more than 15 mm in 72 hours.
- The test may be falsely positive in atypical mycobacterial infection and previous BCG vaccination.
- The test is falsely negative in cutaneous allergy, sarcoidosis, some viral infections, Hodgkin’s disease, recent tuberculosis infection (8 to 10 weeks) and fulminant tuberculosis.
Question 10. Write a short note on syphilis.
Answer:
Syphilis is a venereal (sexually transmitted) disease caused by spirochetes treponema palladium.
T. palladium does not produce any endotoxin and exo- toxin. The pathogenesis of lesions appears to be due to host immune response. Treponema infection is associated with two important antibodies which are:
- The Wasserman antibodies.
- Treponemal antibodies.
Syphilis Mode of Transmission
- Sexual intercourse: Lesion on glans penis, vulva, vagina and cervix.
- Intimate person-to-person contacts lesion on lips, tongue, or fingers.
- Transfusion of infected blood.
- Maternofetal transmission.
Syphilis Stages of Acquired Syphilis
These are primary, secondary and tertiary depending upon the period after which lesion appear and also on the type of lesions.
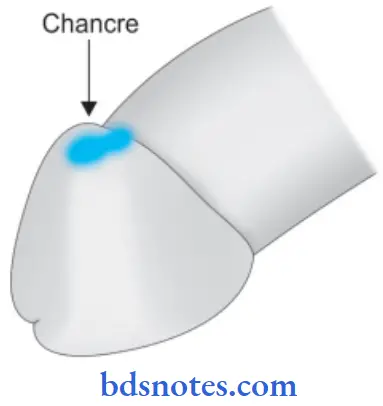
Primary Syphilis
A typical lesion of primary syphilis is chancre which appears on the genitals or at extragenital sites in 2-4 weeks after exposure of infection.
- Initially, the lesion is painless papules which an ulcer ate in the center.
- The fully developed chancre is an indurated lesion with central ulceration accompanied by regional lymphadenitis.
- Chancre heal without scaring.
Histologically
Chancre shows the following features:
- Dense infiltrate of many plasma cells some lymphocytes and few macrophages.
- There is a perivascular aggregation of mononuclear cells mainly plasma cells
- There is the proliferation of vascular endothelium.
Antibody tests are positive in 1 to 3 weeks after chancre is seen.
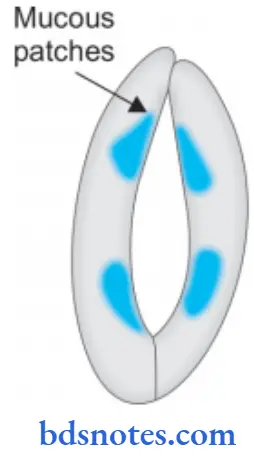
Secondary Syphilis
- Inadequately treated patients of primary syphilis develop mucocutaneous lesions and are painless in 2-3 months after exposure.
- Mucocutaneous lesions may be in form of mucus patches of mouth, pharynx and vagina.
- During this stage antibody tests are positive.
- This stage is highly infective and spirochetes are seen in mucocutaneous lesions.
Tertiary Syphilis
About 2-3 years following first exposure, a tertiary lesion of syphilis appears.
- Lesions of tertiary syphilis are much less infective than the other two stages.

- Lesions of tertiary syphilis are of two types:
1. Syphilitic gumma: It is a localized rubbery lesion with central necrosis seen in the liver, testis, bone and brain.
In liver, it leads to hepar lobatum.
Histologically
Structure of gumma shows:
- Central coagulative necrosis resembles caseation but is less destructive so that outlines of necrosed cells can still be faintly seen.
- Surrounding zone of palisaded macrophages with many plasma cells, some lymphocytes, giant cells and fibroblasts
2. Diffuse lesions of tertiary syphilis: They appear following widespread dissemination of spirochaetes in body. Lesions are seen in the cardiovascular and nervous systems. Lesions are:
- Cardiovascular syphilis: It involves the thoracic aorta. The wall of the aorta get weak and dilated which causes aortic aneurysm, incompetence of the aortic valve and narrowing of coronary ostia.
- Neurosyphilis is manifested as meaning neovascular syphilis in the meninges, tabes dorsalis in the spinal cord and general paresis in the brain.
Diagnostic Tests for Syphilis
The tests are:
1. Nontreponemal antibody tests
- VDRL, i.e. venereal disease research laboratory test
- RPR, i.e. rapid plasma regain test
These tests detect and quantify antibodies to cardiolipin. These tests become positive in 4 to 6 weeks after infection.
These tests are used for screening and for monitoring the response to treatment because they become negative after the therapy.
2. Treponemal antibody tests
- FTA-ABS, i.e. fluorescent treponemal antibody absorption test
- Microhemagglutination assay for T. pallidum antibodies.
These test measure antibodies which specifically react with T. palladium and become positive 4 to 6 weeks after infection, they remain positive even after successful treatment.
Question 11. Write a short note on congenital syphilis.
Answer:
Congenital syphilis may develop in the fetus of more than 16 weeks gestation who is exposed to maternal spiro chaetae mia, there can be three possibilities:
1. Child born dead: The child is premature with macerated skin, enlarged spleen and liver with syphilitic epiphysis.
2. Child born alive: The child may show mucocutaneous lesion of acquired secondary syphilis. The bridge of nose may fall due to ulceration and destruction giving the characteristic ‘saddle nose’ appearance.
3. Late type: Lesions appear after some years. The characteristic ‘Hutchinson’s teeth’ seen in this type are small, widely spaced, peg-shaped permanent teeth.
Morphologic features of congenital syphilis are:
- Saddle-shaped nose deformity due to destruction of bridge of nose.
- Characteristic Hutchinson teeth which are small widely spaced and peg-shaped permanent teeth.
- Mucocutaneous lesion of acquired secondary syphilis.
- Bony lesions such as epiphysitis and periostitis.
- Interstitial keratitis with corneal opacity
- Diffuse firosis in liver
- Interstitial firosis of lungs
- If fetus with congenital syphilis is born dead, it is premature with macerated skin, enlarged spleen and liver with syphilitic epiphysitis.
Question 12. Write a short note on pathology and lab diagnosis of actinomycosis.
Answer:
Pathology of Actinomycosis
Depending on the anatomic location of lesions, antinomy- costs is of four types:
1. Cervicofacial actinomycosis: Infection enters from tonsils, carious teeth, periodontal disease or trauma following tooth extraction.
Initially, a film swelling develops in lower jaw. In time, the mass breaks down and abscesses and sinuses are formed.
The discharging pus contains typical tiny yellow sulphur granules. The infection may extend into adjoining tissues as well as destroy bone.
2. Thoracic actinomycosis: Infection in the lungs is due to aspiration of the organism from oral cavity or extension of infection from abdominal or hepatic lesions.
3. Abdominal actinomycosis: Abdominal infection results from the swallowing of organisms from oral cavity or extension from the thoracic cavity.
4. Pelvic actinomycosis: Infection in the pelvis occurs as a complication of IUCD.

Lab Diagnosis of Actinomycosis
Following are the laboratory diagnosis of actinomycosis.
- Biopsy: Biopsy from the lesional tissue is taken and is assessed microscopically. On microscopic examination, the following features are seen:
- There is the presence of a granuloma with central suppuration.
- There is a formation of abscess in the center of lesion and at the periphery are seen chronic inflammatory cells, giant cells and fibroblasts.
- Center of each abscess contains a bacterial colony ‘sulfur granule’ characterized by radiating filaments with hyaline, eosinophilic, club-like representatives of secreted immunoglobulins.
- Pus from the lesion is collected and sulfur granules are demonstrated by shaking the pus in test tube with saline. Granules are white or yellow-ish in color and measure about 5 mm. On standing granules sediment and are withdrawn with a capillary pipette.
Granules are crushed between the slides and are stained with Gram stain.
Granules are bacterial colonies that consist of thin, Gram-positive filaments surrounded by a peripheral zone of swollen radiating club-shaped structures presenting a sun-ray appearance.
- By Gomori’s methenamine silver stain the organism stain positively.
Question 13. Describe the process of healing in a clean incised wound and mention the factors that may modify the cause of healing.
Or
Write a short note on healing by primary intention.
Or
Discuss in brief the mechanism of wound healing.
Or
Describe the mechanism of healing.
Or
Write a short note on wound healing.
Or
Describe the various factors affecting wound healing.
Answer:
Injury to tissue may result in cell death and tissue necrosis.
- Healing: It is body response to injury in an attempt to restore normal structure and function.
- Healing with first intention or primary union
- Healing of wounds which has the following characteristics.
-
- Clean and uninfected
- Surgically incised
- Without much loss of cells and tissues
- Edges of wounds are approximated by surgical sutures.
Pathology of Actinomycosis Events in Primary Intention
- Initial hemorrhage: Immediately after injury, the space between approximated surfaces of the incised wound is filled with blood which then clots and seals the wound and prevents dehydration and infection.
- Acute inflammatory response: This occurs within 24 hours with the appearance of polymorphs from the margins of the incision. By the third day, polymorphs are replaced by macrophages which are clear of the debris.
- Epithelial changes: The basal cells of the epidermis from both cut margins start proliferating and marginating towards incisional space in the form of epithelial spurs.
- The migrating epithelial cells separate the underlying viable dermis from overlying necrotic material and clot, forming scale which is cast off
- The basal cells from the margins continue to divide. By 5th day a multilayered new epidermis is formed which is differentiated into superficial and deeper layer.
- Organization:
-
- By third day, fibroblasts also include the wound area. By 5th-day new collagen fibrils start forming which are dominant till healing is completed.
- In four weeks, the scar tissue with scanty cellular and vascular elements, a few inflammatory cells and an epithelial surface is formed.
- Suture tracks: Each suture track is a separate wound and incites the same phenomena as in healing of the primary wound, i.e. filing the space with hemorrhage some inflammatory cell reaction, epithelial cell proliferation along the suture track from both margins, fibroblastic proliferation and formation of young collagen.
When sutures are removed around seventh day, much of epithelialized suture track is avulsed and the remaining epithelial tissues in track is absorbed.
However, sometimes the suture track gets infected, or the epithelial cells may persist in the track (implantation or epidermal cysts).
Pathology of Actinomycosis Factors that Modify the Cause of Healing
Two types of factors influencing wound healing.
- Local factors: Those acting locally.
- Infection: It is the most important factor acting locally which delays the process of healing.

-
- Poor blood supply to wound shows delay healing–injuries to face heal quickly due to rich blood supply while injury to leg having poor blood supply heals slowly.
- Foreign Bodies: Including suture interfere with healing and cause intense inflammation reaction and infection.
- Movement delays wound healing.
- Exposure to ionizing radiation delays granulation tissue formation.
- Exposure to UV light facilitates healing.
- Type, size and location of injury determine whether healing takes place by resolution or organization.
- Systemic factors: Those acting in general, these are as under:
- Age: Wound healing is rapid in the young and somewhat slow in aged, due to poor blood supply to the injured area in later.
- Nutrition: The deficiency of constituents like protein, vitamin C, and zinc delays the wound healing.
- Systemic Infection: Delays wound healing.
- The administration of glucocorticoids has anti-inflammatory effects.
- Uncontrolled diabetes are more prone to develop infections and hence delay in healing.
- Hematological abnormalities: Like defects of neutrophil functions, and neutropenia and bleeding disorders slow the process of wound healing.
Question 14. Write a short note on syphilitic gumma.
Answer:
Syphilitic gumma is the lesion of tertiary syphilis. Syphilitic gumma are white-gray and rubbery, occur singly or multiply, and vary in size from microscopic defects resembling tubercles to large tumor-like masses.
- They occur in most organs but particularly in skin, subcutaneous tissue, bone and joints.
- In the liver, scarring of hepatic parenchyma as a result of gummas may cause a distinctive hepatic lesion known as hepar located.
Syphilitic gumma Histologically
On histologic examination, the gummas contain a center of coagulated, necrotic material and margins composed of plump or palisaded macrophages and fibroblasts surrounded by large numbers of mononuclear leukocytes, chiefly plasma cells.
Treponemes are scant in this gumma and are difficult to demonstrate.
Syphilitic gumma In oral cavity
- Gumma can occur anywhere in the oral cavity but the more frequent sites are palate, mandible and tongue.
- It occur as a solitary, deep, punched-out ulcer.
- In gumma breathing and swallowing difficulties are encountered by the patients.
- At times perforation of the palatal wall is present.
- Numerous small healed gumma in the tongue result in series of nodules or sparse in the deeper area giving the tongue an upholstered or tufted appearance.

Question 15. Write a short note on fracture healing.
Answer:
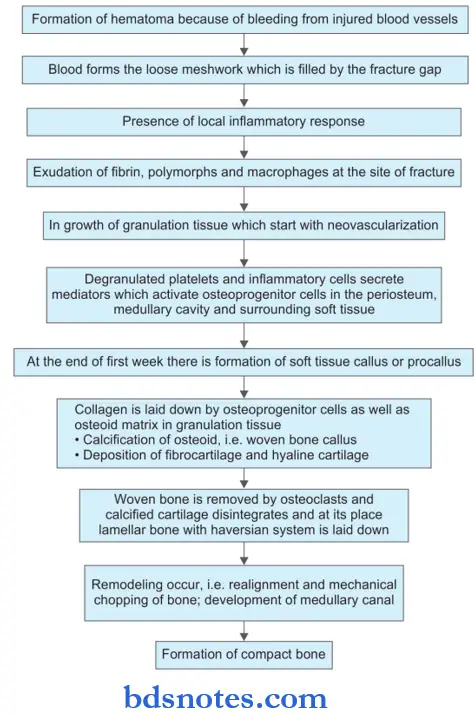
Healing of fracture or repairing of bone occur by callus formation. The process is described as:
Fracture healing Procallus Formation
- Hematoma: It forms due to bleeding from torn blood vessels, filling the area surrounding fracture. Loose meshwork is formed by blood and fibrin clot which act as a framework for subsequent granulation tissue formation.
- Local inflammatory response at the site of injury with exudation of fiers, polymorphs and macrophages, fragments of necrosed bone are scavenged by macrophage and osteoclast.
- Ingrowth of granulation begins with neovascularization and proliferation of mesenchymal cell from the periosteum and endosteum. A soft callus is formed which joins the ends of the fracture bone.
- Callus is composed of woven bone and cartilage. The cells of inner layers of the periosteum have estrogenic potential and lay down collagen as well as osteoid matrix. The osteoid undergoes calcification and is called a woven bone callus. The subperiosteal osteoblast may form cartilage at the fracture site.
Fracture healing Osseous Callus Formation
At their place, newly formed blood vessels and osteoblasts involve, laying down osteoid which is calcified and laminar bone is formed by developing a haversian system concentrically around the blood vessels.
Fracture healing Remodeling
During the formation of lamellar bone, osteoblastic laying and osteoclastic removal are taking place remodeling the united bone ends, which after sometime is indistinguishable from normal bone.
Summary of Healing of Bone

Question 16. Write a short note on possible complications of fracture healing.
Answer:
Possible Complications
- The fibrous union may result instead of the osseous union if the immobilization of fractured bone is not done. Occasionally, a false joint may develop at the fracture site.
- Nonunion may result if some soft tissue is interposed between fracture ends.
- Delayed union may occur from causes of delayed wound healing in general such as infection, inadequate blood supply, poor nutrition movement and old age.
- Pseudoarthrosis: If a nonunion allows too much motion along the fracture gap, central portion of callus undergoes cystic degeneration and its luminal surface become lined by synovial-like cells creating a false joint called pseudoarthrosis.
Question 17. Write a short note on chemotaxis.
Answer:
After extravasation, leukocytes emigrate in tissues to ward the site of injury by a process called chemotaxis.
- All granulocytes, monocytes, and, to a lesser extent, lymphocytes respond to chemotactic stimuli with varying rates of speed.
- Both exogenous and endogenous substances can act as chemoattractants.
- The most common exogenous agents are bacterial products. Some of these are peptides that possess an N-formylmethionine terminal amino acid. Others are lipid in nature.
- Endogenous chemical mediators include components of the complement system, particularly C5a; products of the lipoxygenase pathway, mainly leukotriene B4 (LTB4); and cytokines, particularly those of the chemokine family (e.g. IL-8).
- Binding of chemotactic agents to specific receptors on the cell membranes of leukocytes results in activation of phospholipase C, leading to the hydrolysis of phosphatidylinositol-4,5-biphosphate (PIP2) to inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) and the release of calcium, fist from intracellular stores and subsequently from the influx of extracellular calcium. The increased cytosolic calcium triggers the assembly of contractile elements responsible for cell movement.
- The leukocyte moves by extending a pseudopod (lamellipod) that pulls the remainder of the cell in the direction of extension.
- The interior of the pseudopod consists of a branching network of filaments composed of actin as well as the contractile protein myosin.
- Locomotion involves rapid assembly of actin monomers into linear polymers at the pseudopod’s leading edge, cross-linking of filaments, followed by disassembly of such filaments away from the leading edge. These complex events are controlled by the effcts of calcium ions and phosphoinositol on a number of actin-regulating proteins, such as fiamin, gelsolin, profiin, and calmodulin. These components interact with actin and myosin in the pseudopod to produce a contraction.
- Leukocytes appear to migrate in a step-by-step manner in response to one agonist after another, their position is determined by the pattern of attractant receptors they express, and the sequence of chemokine gradients they encounter.
- In addition, the target-derived chemotaxis overrides the host-derived gradients, helping to guide movement to the initiating stimulus.
The following agents act as potent chemotactic substances for neutrophils:
-
- Leukotriene B4 (LT-B4), a product of the lipooxygenase pathway of arachidonic acid metabolites
- Components of complement system (C5a and C3a in particular)
- Cytokines (interleukins, in particular, IL-8)
- Soluble bacterial products (such as formylated peptides).

Question 18. Describe types of inflammation.
Answer:
Inflammation is defined as a local response of living mammalian tissue to injury due to any agent. It is a body defense reaction in order to eliminate or limit the spread of injurious agent as well as to remove consequent necrosed cells and tissues.
Types of Inflammation
Depending on the defense capacity of the host and duration of response, inflammation can be classified as acute and chronic
Acute inflammation: It is of short duration and represents the early body reaction, resolves quickly and is usually followed by healing.
The main features of acute inflammation are:
- Accumulation of fluid and plasma at the affected site.
- Intravascular activation of platelets
- Polymorphonuclear neutrophils as inflammatory cells.
Sometimes the acute inflammatory response may be quite severe and is termed as fulminant acute inflammation.
Chronic inflammation: It is of longer duration and occurs after delay, either after the causative agent of acute inflammation persists for long time or the stimulus is such that it induces chronic inflammation from the beginning.
A variant, chronic active inflammation is the type of chronic inflammation in which during the course of the disease there are acute exacerbations of activity.
The characteristic feature of chronic inflammation is the presence of chronic inflammatory cells such as lymphocytes, plasma cells and macrophages, granulation tissue formation and in specific situations as granulomatous inflammation.
In some of instances, term subacute inflammation is used for state of inflammation between acute and chronic.
Question 19. Classify chronic granuloma. Describe anyone in detail.
Answer:
Granulomatous Inflammation
Granuloma is defined as a circumscribed tiny lesion, about 1 mm in diameter composed predominantly of collection of modified macrophages called epithelioid cells and rimmed at periphery by lymphoid cells.
Classification of Granulomatous Lesions or Diseases
- Specific or infective type:
- Bacterial:
- Tuberculosis
- Leprosy
- Syphilis
- Granuloma inguinale
- Brucellosis
- Cat scratch disease
- Tularemia
- Glanders
- Actinomycosis.
- Fungal:
- Blastomycosis
- Cryptococcosis
- Coccidioidomycosis
- Histoplasmosis.
- Parasitic: Schistosomiasis.
- Bacterial:
- Non-specific
- Sarcoidosis
- Crohn’s disease
- Silicosis
- Berylliosis
- Foreign body granuloma
- Orofacial granulomatosis.
Question 20. Write a short note on actinomycosis.
Answer:
- Actinomycosis is a chronic suppurative disease caused by the anaerobic bacteria, i.e. Actinomyces israelii.
- The microorganism resides in oral cavity, alimentary tract and vagina.
- Infection is endogenous in origin.
Actinomycosis Morphologic Features
Depending on anatomic location of lesions, actinomycosis is of four types:
1. Cervicofacial actinomycosis: Infection enters from tonsils, carious teeth, periodontal disease or trauma following tooth extraction. Initially, a fim swelling develops in lower jaw. In time, the mass breaks down and abscesses and sinuses are formed. The discharging pus contains typical tiny yellow sulphur granules. The infection may extend into adjoining tissues as well as destroy bone.
2. Thoracic actinomycosis: Infection in the lungs is due to aspiration of organism from oral cavity or extension of infection from abdominal or hepatic lesions.
3. Abdominal actinomycosis: Abdominal infection results from swallowing of organisms from oral cavity or extension from thoracic cavity.
4. Pelvic actinomycosis: Infection in the pelvis occurs as a complication of IUCD.
Actinomycosis Histologically
- On microscopic examination, following features are seen:
- There is the presence ofa granuloma with central suppuration.
- There is formation of an abscess in the center of lesion and at periphery are seen chronic inflammatory cells, giant cells and fibroblasts.
- Center of each abscess contains a bacterial colony ‘sulfur granule’ characterized by radiating filaments with hyaline, eosinophilic, club-like representatives of secreted immunoglobulins.
Question 21. Define and classify inflammation.
Answer:
Inflammation is defined as the local response of living mammalian tissues to injury due to any agent.
It is a body defense reaction in order to eliminate or limit the spread of injurious agent as well as to remove the consequent necrosed cells and tissues.
Classifiation of Inflammation
Depending on the defense capacity of the host and duration of response, inflammation is classified as acute or chronic.
Acute inflammation: It is of short duration and represents the early body reaction, resolves quickly and is usually followed by healing. Sometimes the acute inflammatory response may be quite severe and is termed as fulminant acute inflammation.
Chronic inflammation: It is of longer duration and occurs after delay, either after the causative agent of acute inflammation persists for long time or the stimulus is such that it in- duces chronic inflammation from the beginning. A variant, chronic active inflammation is the type of chronic inflammation in which during the course of the disease there are acute exacerbations of activity.
In some of instances, term subacute inflammation is used for the state of inflammation between acute and chronic.
Question 22. Write a short note on examples of chronic granulomas.
Or
Write briefly on chronic granulomatous inflammation.
Answer:
Granuloma is defined as a circumscribed tiny lesion, about 1 mm in diameter composed predominantly of a collection of modified macrophages called epitheliod cells and rimmed at the periphery by lymphoid cells.
Types of Granulomas
1. Infectious granulomas
- Tuberculosis: It is associated with the formation of caseating granulomas, i.e. granulomas showing presence of central granular debris with loss of all cellular detail and higher positivity for acid fast bacilli or non-caseating granulomas, i.e. absence of caseation and low positivity for acid fast bacilli.
- Leprosy: It is caused by Mycobacterium leprae. Noncaseating granulomas are typically seen with or without acid fast lepra bacilli in the macrophages.
- Syphilis: It is caused by Treponema pallidum. Gumma formation is the disease’s hallmark. Gumma is histopathologically characterized by a central necrotic area without loss of cellular outline; plasma cell infitrate with a wall of histiocytes.
- Cat scratch disease: It is caused by a Gram-negative bacillus. It typically shows rounded or stellate granulomas containing central granular debris and large number of neutrophils.
- Deep fungal infections: Fungal granulomas are caused by organisms like Histoplasma and Blastomyces and are typically suppurative, i.e. granulomas with neutrophilic inflammation.
2. Noninfectious or immune granulomas: Granulomas form in response to the persistent presence of nondegradable or particulate material, which incites an immune response. These are usually non-caseating epithelioid cell granulomas. Examples include sarcoidosis and hypersensitivity pneumonitis.
3. Foreign body granulomas: They are formed as a response to foreign bodies like talc, suture and intravenous drugs. The foreign material can be identified in the center of the granuloma or within the foreign body giant cells which have a haphazard distribution of nuclei unlike Langhans giant cell.
Question 23. Discuss the formation of tubercular granuloma.
Or
Write short note on tubercular granuloma.
Or
Write in brief the granuloma of tuberculosis.
Answer:
Granuloma is a tumor-like proliferation of granulation tissue.
It is seen in granulomatous nodule in rheumatoid arthritis and rheumatic fever.

Formation of Tubercular Granuloma
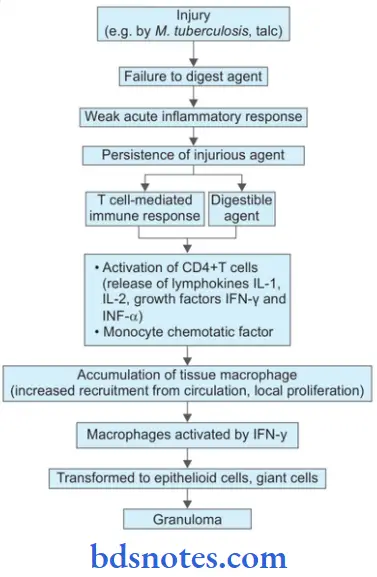
- As the tubercle bacilli are injected intravenously, the bacilli are lodged in pulmonary capillaries where an initial response of neutrophils is evoked which are rapidly destroyed by the organisms. There are two types of cells which are essential for a response to tubercle bacilli, i.e. macrophages and T cells.
- After about l2 hours, there is progressive infiltration by macrophages. This is due to coating of tubercle bacilli with serum complement factors C2a and C3b which act as opsonins and attract the macrophages.
- Macrophages start phagocytosing the tubercle bacilli and either try to kill the bacteria or die away themselves. When macrophages die themselves they produce nitric oxide radicals which have antimycobacterial properties and also Cause increased synthesis of cytokines (TNF-a and IL-1) resulting in the proliferation of macrophages locally as well as increased recruitment from blood monocytes.
- As a part of body’s immune response, T and B cells are activated. Activated CD4+T cells elaborate cytokines, IFN-? and lL-2. These cytokines and their regulators determine the host’s response by infiltrating macrophage monocytes and develop the cell-mediated delayed-type hypersensitivity reaction.
- B cells form antibodies but humoral immunity plays litte role in body’s defense against tubercle bacilli.
- In 2-3 days, the macrophages undergo structural changes as a result of immune mechanisms—the cytoplasm becomes pale and eosinophilic and their nuclei become elongated and vesicular. These modified macrophages resemble epithelial cells and are called epithelioid cells (i.e. epithelial-like).
- Epithelioid cells in time aggregate into tight clusters or granulomas. Release of cytokines in response to sensitized CD4+T cells and some constituents of mycobacterial cell wall play a role in formation of granuloma.
- Some macrophages, unable to destroy tubercle bacilli, fuse together and form multinucleated giant cells. These giant cells may be Langhans type having peripherally arranged nuclei in the form of horseshoe or ring, or clustered at the two poles of the giant cell; or they may be foreign body type having centrally placed nuclei.
- Around the mass or cluster of epithelioid cells and a few giant cells, a zone of lymphocytes and plasma cells is formed which is further surrounded by fibroblasts. The lesion at this stage is called hard tubercle due to the absence of central necrosis.
- Within 10-14 days, the center of the cellular mass begins to undergo caseation necrosis, characterized by a cheesy appearance and high lipid content. This stage is called soft tubercle which is the hallmark of tuberculous lesions.
- The soft tubercle which is a fully-developed granuloma with a caseous center does not favor rapid proliferation of tubercle bacilli.
Question 24. Write the difference between the healing of wounds by primary and secondary intention.
Or
Give differences between primary and secondary healing.
Answer:
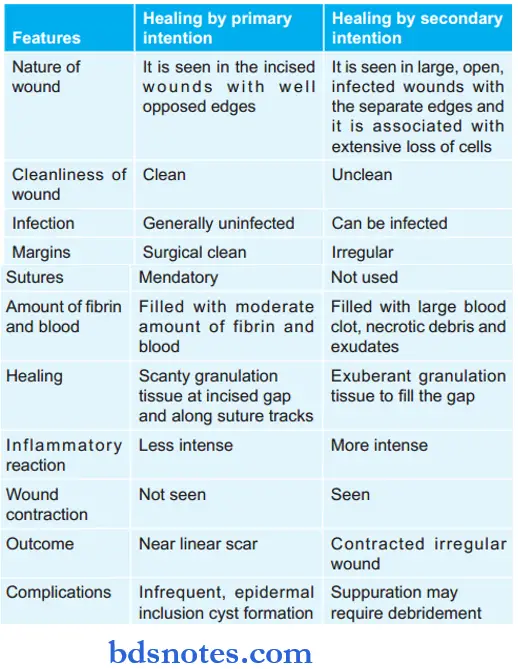
Question 25. Define inflammation and describe events in acute inflammation.
Answer:
Definition: Inflammation is defined as the local response of living mammalian tissues to injury due to any agent.
It is a body defense reaction in order to eliminate or limit the spread of injurious agent as well as to remove the consequent necrosed cells and tissues.
Question 26. Write in detail the morphology, and pathogenicity of M. tuberculosis and write in detail about laboratory diagnosis of pulmonary tuberculosis.
Answer:
Pulmonary tuberculosis Morphology
- It is slender, gram-positive, acid-fast bacilli.
- It is non-sporing, non-capsulated, and non-motile.
- Slightly curved rod with granular or beaded staining.
- It occurs singly, in pairs, in bundles/clumps.
Pulmonary tuberculosis Pathogenicity

Pulmonary tuberculosis Diagnosis
1. Demonstration of AFB on microscopic examination of a diagnostic specimen (sputum or tissue): Smears or tissue slides stained by Ziehl-Neelsen stain are examined for acid fast bacilli. This method has a relatively low sensitivity in confirmed cases of pulmonary tuberculosis. Au-famine-rhodamine staining and fluorescence microscopy can improve the sensitivity to a certain extent. Three sputum specimens preferably collected early in the morning should be submitted to the laboratory for AFB smear and mycobacterial culture.
2. Culture: Besides sputum and tissue other specimens which can be used for culture are body cavity flids, urine or gastric lavage flid. Specimens may be inoculated onto egg or agar-based medium, e.g. Lowenstein-Jensen or Middlebrook 7H10 media and incubated at 37°C. M. tuberculosis grows slowly (4-8 weeks). A presumptive diagnosis can be made based on colony pigmentation and morphology; however, biochemical tests are must for species recognition.
3. Molecular typing: M. tuberculosis is isolated and species identification is done by molecular methods or high-pressure liquid chromatography of mycolic acids (reducing the time required for confirmation to 2-3 weeks). Polymerase chain reaction, i.e. PCR is the conformational method.
4. Tuberculin sensitivity test: It is based on the principle that M. tuberculosis in a concentrated liquid culture medium, i.e. purified protein extract can elicit a skin reaction when injected subcutaneously into patients with tuberculosis. A person is given the tuberculin and asked to return within 48-72 hours to have a trained healthcare worker to look for a reaction on the arm (swelling, induration and erythema) and measure its size. Redness by itself is not considered part of the reaction. The lack of mycobacterial species specificity, subjectivity of interpretation and batch-to-batch variations limit the usefulness of protein-purified derivatives.
5. In vitro assays that measure T cell release of IFN-γ in response to stimulation with the highly tuberculosis-specific antigens ESAT6 and CPP10: These are commercially available assays (Interferon ? release assay or IGRA) IGRAs are more specific than the tuberculin sensitivity test as a result of less cross-reactivity due to BCG vaccination and sensitization by non-tuberculous mycobacteria. IG- RAs also appear to be at least as sensitive as the tuberculin-sensitive test for active tuberculosis.
6. Complete hemogram: It shows lymphocytosis and raised ESR.
7. Fine needle aspiration cytology: This is done in the enlarged peripheral lymph node and is easy way for confirmation of the diagnosis.
8. Immunohistochemistry: Immunohistochemical stain with anti-MBP 64 antibody stain can be used to demonstrate the organism.
Question 27. Write the difference between tuberculoid and lepromatous leprosy.
Or
Write the differences between lepromatous and tuberculoid leprosy.
Answer:
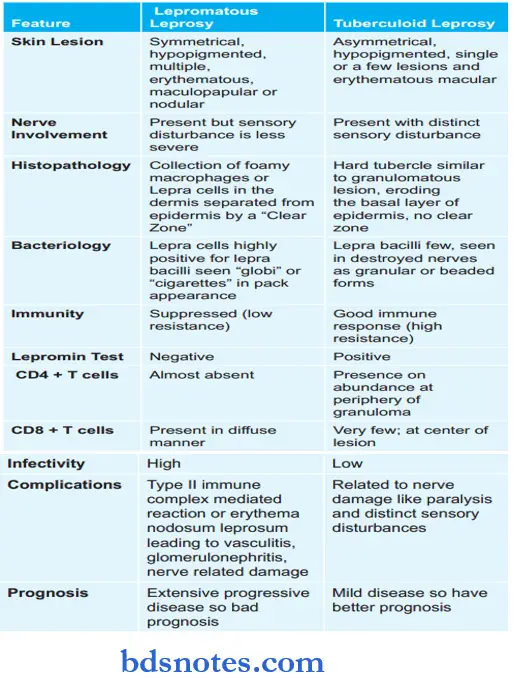
Question 28. Write briefly about secondary pulmonary tuberculosis.
Answer:
The infection of an individual who has been previously infected or sensitized is called as secondary or post-primary or reinfection or chronic tuberculosis.
Secondary Pulmonary Tuberculosis
The lesions in secondary pulmonary tuberculosis usually begin as 1-2 cm apical area of consolidation of lung, which may in time develop a small area of central caseation necrosis and peripheral firosis.
It occurs by the hematogenous spread of infections from primary complex to the apex of affcted lung where the oxygen tension is high and favorable for growth of aerobic tubercle bacilli.
Fate of Secondary Pulmonary Tuberculosis
- The subapical lesion may heal with fibrous scaring and calcification.
- The lesions may coalesce together to form larger area of tuberculosis:
- Fibrocaseous tuberculosis
- Tuberculous caseous pneumonia
- Miliary tuberculosis.
- Tuberculous empyema.
Fibrocaseous Tuberculosis
The original area of tuberculosis pneumonia undergoes massive central caseation necrosis:
- The tubercular cavity is spherical with thick fibrous wall, lined by yellowish, caseous, necrotic material and the lumen is traversed by thrombosed blood vessels.
- Around the wall of cavity foci of consolidation are seen.
- The overlying pleura may also be thickened.
Microscopically
The wall of the cavity shows eosinophilic, granular, caseous material which may show foci of dystrophic calcification.
- Tubercular granulomas consist of epithelioid cells, Langhans giant cells and peripheral mantle of lymphocytes and central caseation necrosis.
- The outer wall of the cavity shows firosis.
Complications
- May produce hemoptysis
- Extending to pleura produces bronchopleural fitula
- Tubercular empyema
- Thickened pleura.
Tuberculous Caseous Pneumonia
In an individual with high degree of hypersensitivity, second- ary pulmonary tuberculosis may spread to rest of the lung, producing caseous pneumonia.
Miliary Tuberculosis
This is the lymphohematogenous spread of tuberculous infection.
- The spread may occur to systemic organ or isolated organs.
- The miliary lesions are millet seed sized (1 mm diameter), yellowish, fim areas.
- Lesions show the structure of tubercles with a minute area of caseation necrosis.
- The spread may be extrapulmonary into liver, spleen, kidney, brain and bone marrow.
Tuberculous Empyema
The caseating pulmonary lesions of tuberculosis may be associated with pleurisy as a reaction and is expressed as a series of fibrinous exudates.
Pleural effusion may heal by fibrosis and obliterate the pleural space. Occasionally the pleural cavity may contain caseous material and develop into tuberculous empyema.
Question 29. Write a short note on tubercular meningitis.
Answer:
Meningitis is inflammatory involvement of the meninges
- Tubercular meningitis is a type of chronic meningitis.
- It causes chronic granulomatous reaction and may produce parenchymal lesions.
- Tuberculous meningitis occurs in children and adults through hematogenous spread of infection from tuberculosis elsewhere in the body or it may simply be a manifestation of military tuberculosis.
- Less commonly the spread may occur directly from tuberculosis of a vertebral body.
Tubercular Meningitis Pathologic Changes
- Grossly the subarachnoid space contains thick exudate particularly abundant in the sulci and the base of the brain.
- Tubercles are 1-2 mm in diameter, may be visible, especially adjacent to blood vessels.
Tubercular meningitis Microscopically
- It shows acute and chronic inflammatory cells and granulomas with or without caseation necrosis and giant cells.
- Acid-fast bacilli may be demonstrated.
- Late case show dense fibrous adhesions in the sub-arachnoid space and consequent hydrocephalus.
Tubercular meningitis Clinical Features
Headache, confusion, malaise and vomiting.
Tubercular meningitis CSF Findings
- Naked eye appearance of a clearer or slightly turbid CSF which may form a firing web on standing.
- Raised CSF pressure (greater than 300 mm water)
- Raised protein content
- Mononuclear leucocytosis
- Lower glucose concentration
- Tubercle bacilli may found on microscopy.
Question 30. Write in brief pyemia and toxemia.
Answer:
Pyemia
It is the dissemination of small septic thrombi in the blood which cause their effcts at the site where they are lodged. This can result in pyemic abscess or septic infarcts.
1. Pyemic Abscess: They are multiple small abscesses in various organs such as in the cerebral cortex, myocardium, lungs and renal cortex.
Pyemic abscess shows a central zone of necrosis containing numerous bacteria surrounded by a zone of suppuration and an outer zone of acute inflammatory cells.
2. Septic Infarcts: It results from the lodgement of large fragments of septic thrombi in the arteries with relatively large foci of necrosis, suppuration and acute inflammation.
Toxemia
It is defined as the distribution throughout the body of poisonous products of bacteria growing in a focal or local site, thus producing generalized symptoms, i.e. fever, diarrhea, malaise, vomiting, quickened or depressed pulse, respiration and shock.
Question 31. Describe the morphology of the etiological agent and laboratory diagnosis of syphilis.
Answer:
Syphilis is a veneral disease caused by spirochetes, Treponema pallidum.
Etiological agent Morphology of Treponema Pallidum
T. pallidum is a coiled spiral filament that is 10 µm long
Etiological agent Laboratory Diagnosis
Syphilis is diagnosed in the laboratory either by demonstration of organism in the specimen by dark fild examination or by a serological test.
Dark Field Examination
Treponema pallidum may be found in the primary lesion or in mucous patches of secondary syphilis. Exudate taken from lesion is examined under dark field microscope. T. pallidum appears as delicate, tightly bound measuring 6 µ.
Serological Tests
They give positive results after 4 weeks of infection. So they are strongly positive in second stage and in congenital syphilis.
Syphilis produces two types of antibodies, i.e. specific Antitreponemal antibodies and non-special reagin antibodies which are measured by specific and non-specific tests.
Etiological agent Non-Specific
In these test, flocculation of antigen suspension occurs and is seen.
The VDRL Test
- Patients infected with T. pallidum produce a nonspecific antibody-like substance called reagin. When the VDRL antigen mixture made of cardiolipin, cholesterol and lecithin is reacted with serum containing regain visible reaction, flocculation, occurs.
- The test is reactive in 70-99% of primary and secondary syphilis cases but is usually non-active in tertiary cases.
The RPR Test
- The RPR test has a carbon-containing cardiolipin antigen that reacts with the antibody-like substance produced in response to syphilis and other conditions.
- This is also a flocculation test, with the carbon causing black clumps at the white background in a reactive test. Test results are reported as ‘reactive’ or ‘non-reactive.”
- A reactive result does not mean the patient definitely has syphilis; it only indicates the presence of time nonspecific antibodies.
Etiological agent Specific Test
- Hemagglutination test such as T. pallidum hemagglutination assay
- Fluorescent test such as florescent T. pallidum antibody absorption
- Immobilization test, i.e. Treponema pallidum immobilization.
- Treponemal ELISA test.
Etiological agent Other Test
Chest skiagram for calcification of aorta in cardiovascular syphilis
CSF examination in neurosyphilis.
Question 32. Write in brief about tissue regeneration.
Answer:
Regeneration
When healing takes place by the proliferation of parenchymal cells and usually results in the restoration of original tissues.
- In order to maintain the proper structure of tissues, cells are under constant regulatory parenchymal control of their cell cycle.
- Cell cycle is defined as the period between two successive cell divisions and is divided into unequal phases.
- G1 (Pre-Mitotic Gap) Phase: It is the stage when mRNA for proteins and proteins themselves required for DNA synthesis are synthesized.
- S Phase: During this phase synthesis of nuclear DNA takes place.

- G2 Phase: It is the short gap phase in which the correctness of DNA synthesized is assessed.
- M Phase: It is the stage in which process of mitosis to form two daughter cells is completed, this occurs in four sequential stages, i.e. prophase, metaphase, anaphase and telophase.
- Prophase:
- Each chromosome divides into two chromatids which are held together by a centromere.
- The centriole divides and two daughter centrioles form the spindle, while the chromosomes line up at the equatorial plate of the spindle.
- Anaphase:
- The centromeres divide and each set of separated chromosomes moves towards the opposite poles of the spindle.
- Cell membrane also begins to divide.
- Telophase:
- There is the formation of a nuclear membrane around each set of chromosomes and the reconstitution of the nucleus.
- The cytoplasm of the two daughter cells completely separates.
- Go Phase: The daughter cells may continue to remain in the cell cycle and divide further or may go out of the cell cycle into the resting phase called as G0 phase.
- Prophase:
Regeneration of parenchymal cells involves the following two processes:
- The proliferation of original cells from the margin of injury with migration so as to cover the gap.
- The proliferation of migrated cells with subsequent differentiation and maturation so as to reconstitute the original tissue.
Question 33. Write a note on the reaction of the body to injury.
Answer:
Body response to injury is healing.
The process of healing involves:
1. Regeneration: When healing takes place by the proliferation of parenchymal cells and usually results in complete restoration of original tissues.
2. Repair: When the healing takes place by the proliferation of connective tissue elements, this result in firosis and scaring.
Two processes are involved in the repair:
- Granulation Tissue Formation
- Contraction of Wounds.
Granulation Tissue Formation
Granulation tissue is the proliferation of new small blood vessels that are slightly lifted on surface by a thin covering of fibroblasts and young collagen.
The following 3 phases are observed in the formation of granulation tissue:
- The phase of inflammation: Following trauma, blood clots at the site of injury. There is an acute inflammatory response with the exudation of plasma, neutrophils and some monocytes.
- The phase of clearance: Combination of proteolytic enzymes liberated from neutrophils, autolytic enzymes from dead Tissues cells, and phagocytic activity macrophages clear of the necrotic tissue, debris and blood cells.
- The phase of in the growth of granulation tissue: This phase consists of 2 main processes—angiogenesis or neovascularization and fibrogenesis
- Angiogenesis: The formation of new blood vessels at the site of injury takes place by the proliferation of endothelial cells from the margins of severed blood vessels.
Initially, the proliferated endothelial cells are solid buds but within a few hours develop a lumen and start carrying blood. The newly formed vessels are more Leaky accounting for the edematous appearance of new granulation tissue. Soon, these blood vessels differentiate into muscular arterioles, thin-walled venules and true capillaries. - Fibrogenesis: The newly formed blood vessels are present in an amorphous ground substance or matrix. The new fibroblasts originate from fibrocytes as well as by mitotic division of fibroblasts.
Some of these fibroblasts have a combination of morphologic and functional characteristics of smooth muscle cells known as myofibroblasts.
- Angiogenesis: The formation of new blood vessels at the site of injury takes place by the proliferation of endothelial cells from the margins of severed blood vessels.
Collagen fibrils begin to appear by about 6th day. As maturation proceeds, more and more of collagen is formed while the number of active fibroblasts and new blood vessels decreases. This results in the formation of an inactive-looking scar known as cicatrization.
Contraction of Wound
The wound starts contracting after 2-3 days and the process is completed by the 14th day. During this period, the wound is reduced by approximately 80% of its original size. Contracted wound results in rapid healing since a lesser surface area of the injured tissue has to be replaced.
Question 34. Write a note on oral lesions of syphilis.
Answer:
Oral Lesions of Syphilis
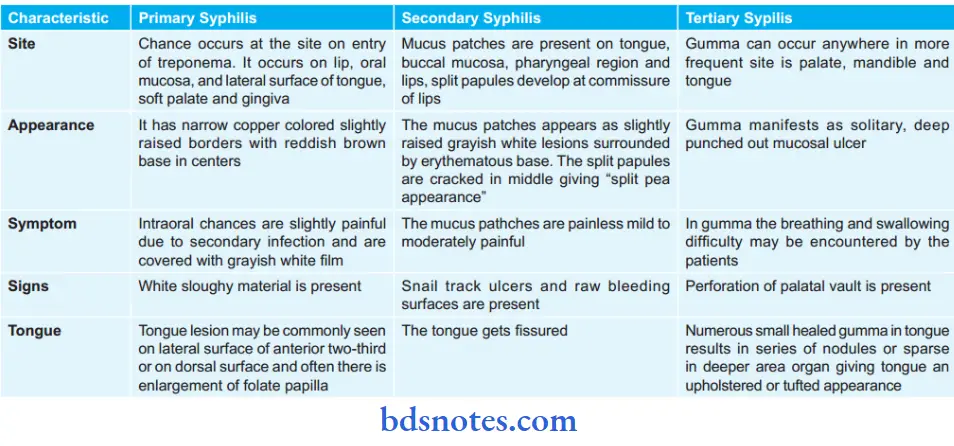
Question 35. Write a short note on chronic inflammation.
Answer:
Chronic inflammation is defined as the prolonged process in which tissue destruction and inflammation occur at the same time.
Various Ways of Chronic Inflammation
- Chronic inflammation following acute inflammation. When the tissue destruction is extensive, and the bacteria survive in small numbers at site of acute inflammation.
- Recurrent attacks of acute inflammation.
- Chronic inflammation starts when an infection with an organism of low pathogenicity occurs.
Morphologic Features of Chronic Inflammation
- Mononuclear cell infiltration: Chronic inflammatory lesions are infiltrated by mononuclear inflammatory cells like phagocytes and lymphoid cells. Other chronic inflammatory cells include lymphocytes, plasma cells, eosinophils and mast cells.
- Tissue destruction or necrosis: This is brought about by activated macrophages with the release of a variety of biologically active substances, e.g. protease, elastase, collagenase, cytokines, etc.
- Proliferative changes: As a result of necrosis proliferation of small blood vessels and fibroblasts is stimulated resulting in the formation of inflammatory granulation tissue.
Systemic Effects of Chronic Inflammation
- Fever: Mild fever with loss of weight and weakness
- Anemia
- Leucocytosis: There is relative lymphocytosis
- ESR: It is elevated in chronic inflammation
- Amyloidosis: Long-term cases of chronic suppurative inflammation may develop secondary systemic amyloidosis.

Types of Chronic Inflammation
- Nonspecific: There is a non-specific chronic inflammatory reaction with the formation of granulation tissue and healing with fibrosis, For Example. chronic osteomyelitis, chronic ulcer.
- Specific: When there is a characteristic histologic tissue response, For Example. tuberculosis and syphilis.
Cells involved in Chronic Inflammation
- Lymphocytes: They are mobilized in antibody-mediated, cell-mediated as well as non-immune inflammation.
- Macrophages: They cause phagocytosis, initiate tissue repair, secrete mediators of inflammation and influence lymphocyte function.
- Eosinophil: They leads to recruitment and extravasation from blood driven by adhesion molecules like neutrophils and by eotaxin. These cells are also involved in IgE-mediated immune reactions and parasitic infestations. Major basic protein is a highly toxic protein contained in eosinophil granules. This protein is toxic to parasites and mammalian epithelial cells.
- Mast cells: They are present in connective tissue. They participate in acute and chronic inflammation. They express on their surface the receptor which binds to the Fc portion of the IgE antibody to degranulate mast cells and release mediators.
- Plasma cells: These are large cells with amphophilic to basophilic cytoplasm, an eccentric nucleus with chromatin arranged in a characteristic cart-wheel or clock-face pattern. The cytoplasm of a cell has a pale perinuclear zone which on electron microscopy shows extensive Golgi apparatus and centrioles. These cells have abundant rough endoplasmic reticulum which is coupled by well developed Golgi apparatus, which together are responsible for immunoglobulin secretion.
- Neutrophils: They are classically associated with acute inflammation, but sometimes are seen with chronic inflammation, For Example. chronic osteomyelitis
Question 36. Enumerate types of inflammation.
Answer:
Types of Inflammation
- Acute Inflammation
- Chronic Inflammation
- Subacute Inflammation.
Question 37. Describe pathological features of syphilis.
Answer:
Pathological Features of Syphilis
Primary Syphilis
The chancre histologically presents the following features:
- Proliferative granulation tissue is present at the margin of the ulcer.
- Dense infiltrates of plasma cells, lymphocytes and macrophages.
- Obliterative endarteritis with perivascular infiltration of chronic inflammatory cells.
- T. pallidum may be seen when immunofluorescent studies or silver staining are done.
Secondary Syphilis
- The macular lesion shows inflammatory cell infiltration and obliterative endarteritis.
- The papular lesion exhibits endothelial proliferation, swelling, and perivascular chronic inflammatory cell infiltration.
- Condyloma lata reveals hyperplastic epithelium with hyperkeratosis and acanthosis.
- Obliterative endarteritis may also be seen and there may be occasional presence of epitheloid cells.
Tertiary Syphilis
- The gumma microscopically presents a peripheral rim made up of fibroblasts, which surrounds a central zone of coagulative necrosis.
- Fibroblasts are plump and they often resemble epithelioid cells.
- The occasional presence of giant cells and the regular presence of chronic inflammatory cells like plasma cells, lymphocytes, histiocytes, etc.
Question 38. Describe the pathogenesis of tuberculosis.
Answer:
Pathogenesis of Tuberculosis
- The interaction of bacilli and the host begins when droplet nuclei from infectious patients are inhaled.
- The majority of the bacilli are trapped and exhaled by ciliary action and a fraction less than 10% enters the alveoli.
- In the initial stage of host-bacterial interaction, either the host’s macrophages control the multiplication of the bacteria or the bacteria grow and kill the macrophages.
- Non-activated monocytes attacked from the bloodstream to the site by various chemotactic factors ingest the bacilli released from the lysed macrophages.
- Initial stages are asymptomatic; about 2-4 weeks after infection tissue damaging and macrophage activating responses develop.
- With the development of specific immunity and accumulation of a large number of activated macrophages at the site of the primary lesion, granulomatous reactions or tubercles are formed.
- The hard tubercle consists of epithelioid cells, Langhans giant cells, plasma cells, and fibroblasts. These lesions develop when host resistance is high.
- Due to cell-mediated immunity in the majority of individuals, local macrophages are activated and lymphokines are released, which neutralize the bacilli and prevent further tissue destruction.
- The central part of the lesion contains caseous, soft, and cheesy necrotic material (caseous necrosis). This necrotic material may undergo calcification at a later stage called
- Ranne complex, in the lung parenchyma and hilar lymph nodes in few cases.
- Caseous necrotic material undergoes liquefaction and discharges into the lungs leading to the formation of a cavity. Spontaneous healing of the cavity occurs either by fibrosis or collapse.
- Calcification of the cavities may occur in which bacteria persist.
- In the early stages, the spread of infection is mainly by macrophages to lymph nodes, other tissues and organs: However, in children with poor immunity hematogenous spread results in fatal miliary TB or tuberculous meningitis.
Question 39. Write a short note on tuberculosis.
Or
Write in short on tuberculosis.
Or
Write a short answer on tuberculosis.
Answer:
Tuberculosis is a granulomatous infection in humans: Tuberculosis is caused by Mycobacterium tuberculosis in lungs and other tissues of human body.
Spread of Tuberculosis
- Local spread: Macrophages cause it by carrying bacilli in surrounding tissues.
- Lymphatic spread: Tuberculosis is primarily an infection of lymphoid tissues. Bacilli may pass into lymphoid follicles of the pharynx, bronchi, intestines or regional lymph nodes leading to regional tuberculous lymphadenitis which is typical of childhood infections. The primary complex is the primary focus with lymphangitis and lymphadenitis.
- Hematogenous spread: It occurs either as a result of tuberculous bacteremia due to drainage of lymphatics into the venous system or because of caseous material escaping via an ulcerated wall of vein. This produces millet seed-sized lesions in diffrent organs of the body like lungs, liver, kidneys, bones and other tissues and is known as miliary tuberculosis.
- By natural passages: Infection can spread from:
- Lung lesions into pleura (tuberculous pleurisy)
- Transbronchial spread into the adjacent lung segments
- Tuberculous salpingitis into the peritoneal cavity (tuberculous peritonitis)
- Infected sputum into the larynx (tuberculous laryngitis)
- Swallowingofinfectedsputum(ileocaecal tuberculosis)
- Renal lesions into the ureter and down to the trigone of the bladder.
Types of Tuberculosis
Depending on type of tissue response and age, infection of tubercle bacilli is of two types:
- Primary tuberculosis
- Secondary tuberculosis
Primary Tuberculosis
- Infection of an individual who was not previously infected or immunized is known as primary tuberculosis or Ghon’s complex or childhood tuberculosis.
- The primary complex is the lesion that is produced inside the tissue of the portal of entry with foci in the draining lymphatic vessels and lymph nodes.
- Most commonly involved tissues for the primary complex are the lungs and hilar lymph nodes. Other tissues which may show primary complexity are tonsils and cervical lymph nodes, and in the case of ingested bacilli, the lesions may be found in the small intestine and mesenteric lymph nodes.
- Incidence of the disseminated form of progressive primary.
- Tuberculosis is particularly high in immunocompromised host, For Example. in patients of AIDS.
- Primary tuberculosis in the lungs have three components:
- Pulmonary component: The lesion inside the lung is the primary focus or Ghon’s focus. It is usually a 1-2 cm solitary area of tuberculous pneumonia which is located peripherally under a patch of pleurisy, in any part of the lung but more often in subpleural focus in upper part of the lower lobe.
- Lymphatic vessel component: Lymphatics draining the lung lesion consists of phagocytes which contain bacilli and may develop beaded, miliary tubercles along the path of hilar lymph nodes.
- Lymph node component: This consists of enlarged hilar and tracheobronchial lymph nodes in the area drained. The affected lymph nodes are matted and show caseation necrosis. Nodal lesions are a potential source of reinfection later.
Secondary Tuberculosis
It is the infection of an individual who has been previously infected or sensitized and is known as secondary or postprimary reinfection, or chronic tuberculosis. Infection may occur from:
- Endogenous source such as reactivation of dormant primary complex
- Exogenous source such as fresh dose of reinfection by tubercle bacilli.
Secondary tuberculosis occurs most commonly in lungs. Other sites and tissues which can be involved are lymph nodes, tonsils, pharynx, larynx, small intestine and skin.
Diagnosis of Tuberculosis
Diagnosis is made by the following tests:
- AFB microscopy of diagnostic specimens such as sputum, and aspirated material.
- Mycobacterial culture: Traditional method on LJ medium for 4-8 weeks, the newer rapid method by HPLC of mycolic acid with result in 2-3 weeks.
- Molecular methods such as PCR.
- Complete hemogram: Presence of lymphocytosis and raised ESR.
- Radiographic procedures, For Example. chest X-ray showing characteristic hilar nodules and other parenchymal changes.
- Mantoux skin test.
- Serologic tests based on the detection of antibodies are not useful although these are being advocated in some developing countries.
- Fine needle aspiration cytology of an enlarged peripheral lymph node is a quite useful and easy way for confirmation of diagnosis and has largely replaced the biopsy diagnosis of tuberculosis.
Question 40. Write a short note on diphtheria.
Answer:
Diphtheria is caused by a slender gram-positive rod, Corynebacterium diphtheriae.
- It is passed from person to person through aerosols or skin shedding.
- C. diphtheriae causes a range of illnesses: asymptomatic carriage; skin lesions in neglected wounds of combat troops in the tropics; and a life-threatening syndrome that includes the formation of a tough pharyngeal membrane and toxin-mediated damage to the heart, nerves and other organs:
Diphtheria Pathogenesis
Dipthteria releases toxins and produces local and systemic effects.
Diphtheria Local Effects
The bacilli remain confined to the site of entry where they multiply and start producing toxins. The toxin causes local necrotic changes along with superficial inflammatory reactions.
The necrosed epithelium together with fibrinous exudate, leucocytes, erythrocytes and bacteria, constitute the pseudomembrane, which is a characteristic feature of diphtheritic infection.
The mechanical complications of diphtheria are due to pseudomem- brane, whereas the systemic effcts are due to toxins.
Diphtheria Systemic Effects
Diphtheria toxin diffuses into the bloodstream and causes toxemia. The toxin has got an affinity for cardiac muscle, adrenals and nerve endings.
It acts systemically on the cells of these tissues. The bacilli themselves do not play any part in systemic effects because they neither penetrate into the tissues nor pass as into the bloodstream producing bacteremia.
Diphtheria Laboratory Investigations
Isolation of Organism
- Collection of specimen: Two swabs from the lesions (throat, nose, larynx, ear, conjunctiva, vagina, or skin) are Collected. One swab is used for smear examination and the other for culture.
- Direct microscopy: Smears are stained with both Gram and Albert stains. Diphtheria bacilli show beaded slender green rods in a typical Chinese letter pattern on Albert’s staining. In Gram staining, they resemble Gram-positive organisms.
- Culture: The swabs are inoculated on the following culture media:
-
- Loeffl’s serum slope: Growth appears within 6-8 hours on this medium. Subculture from Loeffl’s serum slope is made on tellurite blood agar and slate is incubated at 37°C for 48 hours.
- Tellurite blood agar: These plates have to be incubated at 37°C for at least 48 hours before declaring these as negative, as growth may sometimes be delayed.
- Blood agar: It is useful for differentiating streptococcal or staphylococcal pharyngitis, which may simulate diphtheria.
- Colony morphology and staining: On Loeffl’s serum slope, the colonies are small, circular, white or creamy. Diphtheria bacilli grow as black or gray-colored colonies on tellurite blood agar. Smears are prepared from suspected growth from various media. These smears are stained with Albert and Gram stains to confirm the morphology of C. diphtheriae. Albert staining shows green bacilli with bluish-black metachromatic granules. Gram staining reveals Gram-positive bacilli.
- Biochemical reactions: Hiss’s serum water is used for testing the fermentation of carbohydrates.
- Elek’s gel precipitation test: This is an immuno-diffusion test. A rectangular strip of filter paper soaked in diphtheria antitoxin (1000 units per ml) is placed on the surface of a 20% horse serum agar plate while the medium is still fluid.
When the agar solidifies, the test strain is streaked at right angle to the filter paper strip. The positive and negative controls are also put up. The plate is incubated at 37°C for 24 to 48 hours.
The toxin produced by the bacterial growth diffuses in the agar and produces a line of precipitation where it meets the antitoxin at optimum concentration.

Leave a Reply