Immunopathology Including Amyloidosis
Question 1. Write a short note on laboratory diagnosis of AIDS.
Answer:
Laboratory Diagnosis of AIDS
1. Tests for Establishing HIV Infection
- Antibody tests:
- ELISA
- Western blot
- Direct detection of HIV
- p24 antigen capture assay
- HIV RNA assay `
- NA—PCR
- Culture of HIV
2. Tests for Defects in Immunity
- CD4+ T cell count: Fall
- CD8+ cell count: Increased
- Ratio of CD4+ T cell/CD8+T cell count: Reversed
- Lymphopenia
- Hypergammaglobulinemia
- Increased β2 microglobulin level
- Platelet count: Thrombocytopenia
Read And Learn More: Pathology Question And Answers
3. Tests for Detection of Opportunistic Infection and Secondary Tumors
- FNAC/exfoliative cytology
- Biopsy.
1. Tests for Establishing HIV Infection
These include antibody tests and direct detection of HIV
- Antibody tests: These tests are as under:
- ELISA:
- Initial screening is done by a serologic test for antibodies by enzyme-linked immunosorbent assay (ELISA) against gag and env proteins.
- The term window period is used for the initial 2 to 4 weeks period when the patient is infectious but the screening test is negative, while seroconversion is the term used for the appearance of antibodies.
- Western blot: If ELISA is positive, confirmation is done by Western blot for the presence of specific antibodies against all three HIV antigens: gag, pol, and env.
- Direct detection of HIV: These tests are as follows:
- p24 antigen capture assay
- HIV/RNA assay methods by reverse transcriptase (RT) PCR, branched DNA, and nucleic acid sequence-based amplification (NucliSens).
- DNA-PCR by amplification of proviral DNA.
- Culture of HIV from blood monocytes and CD4+ T cells.
- ELISA:
2. Tests for Defects in Immunity
These tests are used for diagnosis as well as for monitoring the treatment of cases.
- CD4+ T cell counts. A progressive fall in the number of CD4 + T cells is of paramount importance in diagnosis and staging CDC categories as described above.
- Rise in CD8+ T cells.
- Reversal of CD4+ to CD8+ T cell ratio.
- Presence of Lymphopenia.
- Polyclonal hypergammaglobulinaemia.
- Increased β2 microglobulin levels.
- Platelet counts reveal thrombocytopenia.
3. Tests for Detection of Opportunistic Infections and Secondary Tumors
Diagnosis of organs involved in opportunistic infection and specific tumors secondary to HIV/AIDS is made by aspiration or biopsy methods as for the corresponding primary disease.
Lymph Node Biopsy
In Early Stage
- Presence of marked follicular hyperplasia
- Follicles extend to the medulla and sometimes spread outside the capsule
- The mantle zone thinned out and germinal centers seem to merge with the interfollicular areas
- Presence of mono cytoid B cells in and around sinusoids and in trabecular blood vessels.
- Involvement of the B cell areas of the lymph node supports polyclonal B cell activation and hypergammaglobulinemia
During Disease Progression
- Presence of severe follicular involution.
- Follicles are depleted of cells.
- An organized network of follicular dendritic cells is disrupted.
- Germinal centers become hyalinized
- Atrophic and small lymph nodes.
- In this stage, lymph nodes may harbor opportunistic infections.
- The inflammatory response to infections in both nodal and extranodal sites may be atypical and sparse.
Question 2. Write A Short Note On Preventive Measures For Aids.
Answer:
Practice safer sex: This includes using a condom unless you are in a relationship with one partner who does not have HIV or other sex partners:
- Never share intravenous (IV) needles, syringes, cotton, cocaine spoons, or eyedroppers with others if you use drugs.
- Do not donate blood, plasma, semen, body organs, or body tissues.
- Do not share personal items, such as toothbrushes, razors, or sex toys, that may be contaminated with blood, semen, or vaginal fluids.
- The risk of a woman spreading HIV to her baby can be greatly reduced if she is on medicine that reduces the amount of virus in her blood to undetectable
levels during pregnancy; continues treatment during pregnancy; does not breastfeed her baby. - Healthcare workers should take universal precautions while treating HIV-positive patients.
Question 3. Write in short about hypersensitivity.
Or
Write a brief on hypersensitivity reactions.
Or
Describeinbriefaboutdiffrenttypeofhypersensitivity reactions.
Or
Write a short note on hypersensitivity reactions.
Answer:
Hypersensitivity refers to a condition in which the immune response results in excessive reactions which leads to tissue damage, disease, or even death in the sensitized host.
Classification of Hypersensitivity Reactions
Hypersensitivity reactions are classified into four major types by Coomb & Gel (1963).
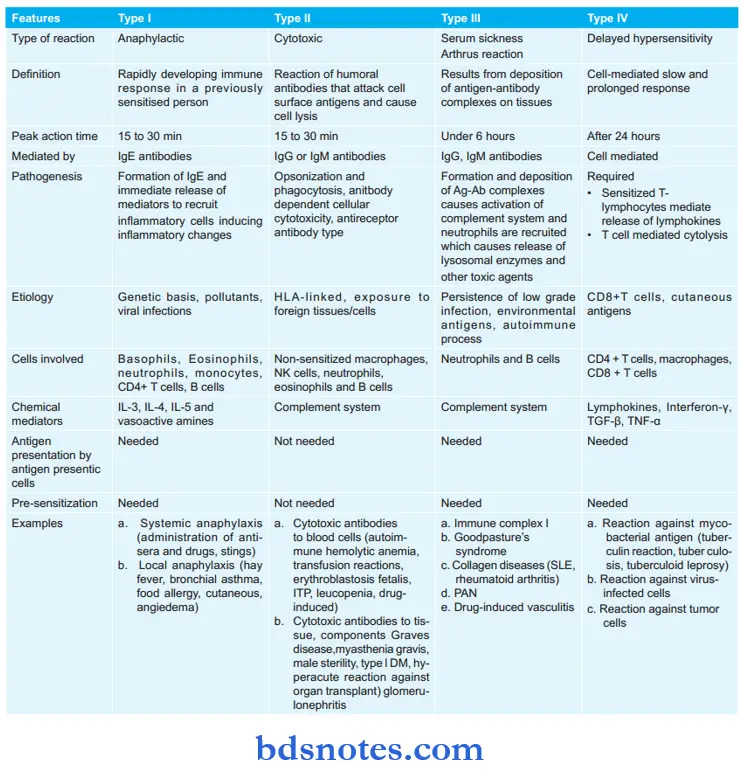
- Type 1: Hypersensitivity
- Type 2: Cytotoxic
- Type 3: Immune complex
- Type 4: Delayed or cell-mediated
- Type 1, 2, and 3 depend on the interaction of antigen with humoral antibodies and are known as immediate type reactions while Type 4 is mediated by T-lymphocytes and is known as delayed hypersensitivity.
Later on, Type 5 hypersensitivity reaction was also described, i.e. stimulatory type.
Type 5: Stimulatory Type
It is the modification of the Type 2 hypersensitivity reaction. Antibodies react with antigens over the cell surface which causes cell proliferation and differentiation in place of killing or inhibition. Antigen–antibody reaction enhances the activity of affected cells.
Question 4. Write A Short Note On Anaphylaxis.
Or
Write a Short Note On Type I Hypersensitivity Reaction.
Answer:
Type 1 hypersensitivity reaction is also known as anaphylaxis or anaphylactic reaction.
Type 1 hypersensitivity is defined as a state of rapidly developing or anaphylactic type of immune response to an antigen to which the individual is previously sensitized. The reaction appears within 15 to 30 min of exposure to an antigen.
Types of Anaphylaxis
- Local (atopy): It occurs when the antigen is confined to a particular site. It manifests with skin allergy, hives, nasal and conjunctival discharge, etc. It has two distinct phases, i.e. immediate and late phase.
- Systemic: It mostly follows parenteral administration but can also result from the ingestion of an allergen. It includes itching, erythema, contraction of respiratory bronchioles, diarrhea, pulmonary edema, pulmonary hemorrhage, shock, and death.
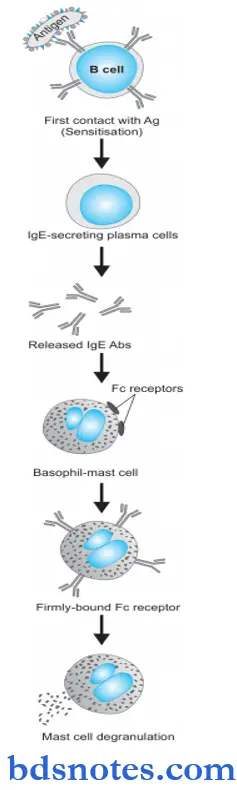
Anaphylaxis Pathogenesis
Type 1 reaction includes participation by B lymphocytes and plasma cells, mast cells and basophils, neutrophils, and eosinophils. The underlying mechanism is as follows:
- During the first contact of the host with antigen, sensitization takes place. In response to initial contact with antigen, circulating B lymphocytes get activated and differentiate to form IgE-secreting plasma cells. IgE antibodies so formed bind to the Fc receptors present in plenty on the surface of mast cells and basophils, which are the main effector cells of type I reaction. Thus, these cells are now fully sensitized for the next event.
- During the second contact with the same antigen, IgE antibodies on the surface of mast cells—basophils are so firmly bound to Fc receptors that it sets in cell damage i.e. there is membrane lysis, influx of sodium and water and degranulation of mast cells, and basophils.
- Released granules contain important chemicals and enzymes with proinflammatory properties histamine, serotonin, vasoactive intestinal peptide (VIP), chemotactic factors of anaphylaxis for neutrophils and eosinophils, leukotrienes B4 and D4, prostaglandins (thromboxane A2, prostaglandin D2, and E2) and platelet-activating factor.
The effects of these agents are:
- Increased vascular permeability
- Smooth muscle contraction
- Early vasoconstriction followed by vasodilatation
- Shock
- Increased gastric secretion
- Increased nasal and lacrimal secretions
- Increased migration of eosinophils and neutrophils at the site of local injury as well as their rise in the blood (eosinophilia and neutrophilia).
Examples of Type 1 Reaction
Based on the types of Type 1 hypersensitivity reaction, i.e. systemic or local anaphylaxis the examples are:
Systemic Anaphylaxis
- Administration of antisera, For Example. anti-tetanus serum (ATS).
- Administration of drugs, For Example. penicillin.
- Sting by a wasp or bee.
Local Anaphylaxis
- Hay fever (seasonal allergic rhinitis) due to pollen sensitization of conjunctiva and nasal passages.
- Bronchial asthma due to allergy to inhaled allergens like house dust.
- Food allergy to ingested allergens like fish, cow’s milk, eggs, etc.
- Cutaneous anaphylaxis due to contact of antigen with skin characterized by urticaria, wheal, and flare.
- Angioedema is an autosomal dominant inherited disorder characterized by laryngeal edema, and edema of eyelids, lips, tongue, and trunk.
Question 5. Write a brief note on Type 2 hypersensitivity reaction.
Answer:
Type 2 hypersensitivity reaction is also known as antibody-mediated reaction or cytotoxic reaction.
It is defined as a reaction by humoral antibodies that attack cell surface antigens on the specific cells and tissues and cause lysis of target cells. Type 2 reaction too appears generally within 15-30 minutes after exposure to an antigen but in myasthenia gravis and thyroiditis, it may appear after a long duration.
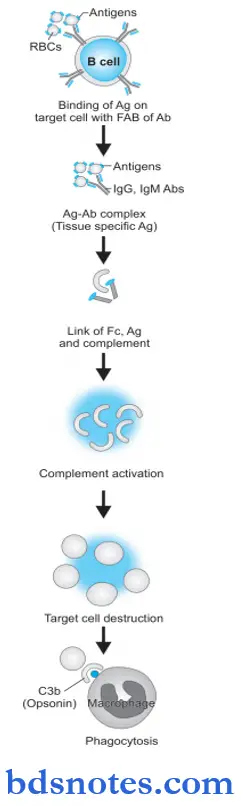
Type 2 hypersensitivity Pathogenesis
Type 2 reactions participate by the complement system, tissue macrophages, platelets, natural killer cells, neutrophils, and eosinophils while the main antibodies are IgG and IgM class.
Type 2 hypersensitivity is tissue-specific and the reaction occurs after antibodies bind to tissue-specific antigens, most often on blood cells.
The mechanism involved is as under:
- The antigen on the surface of the target cell (foreign cell) attacks and binds the Fab portion of the antibody (IgG or IgM) forming the antigen-antibody complex.
- The unattached Fc fragment of antibodies (IgG or IgM) forms a link between the antigen and complement.
- The antigen-antibody binding with forming link causes activation of the classical pathway of serum complement which generates activated complement component, C3b by splitting C4 and C2 by C1.
- Activated C3b bound to the target cell acts as an opsonin and attacks phagocyte at the site of cell injury and initiates phagocytosis.
- Antigen—antibody complex also activates the complement system and exposes membrane attack complex (MAC i.e. C5b—C9) that attacks and destroys the target cell.
Examples of Type 2 Hypersensitivity Reaction
Examples of type 2 reactions are mainly on blood cells and some other body cells and tissues.
1. Cytotoxic Antibodies to Blood Cells
These are more common. Some examples are as under:
- Autoimmune hemolytic anemia in which the red cell injury is brought about by
autoantibodies reacting with antigens present on the red cell membrane. The antiglobulin test (direct Coombs test) is employed to detect the antibody on the red cell surface. - Transfusion reactions due to incompatible or mismatched blood transfusion.
- Hemolytic disease of the newborn i.e. erythroblastosis fetalis in which the fetal red cells are destroyed by maternal isoantibodies crossing the placenta.
- Immune thrombocytopenic purpura is the immunologic destruction of platelets by autoantibodies reacting with the surface components of normal platelets.
2. Cytotoxic Antibodies to Tissue Components
Cellular injury may be brought about by autoantibodies reacting with some components of tissue cells in certain diseases.
- In Graves’ disease (primary hyperthyroidism), thyroid autoantibody is formed which reacts with the TSH receptor to cause hyperfunction and proliferation.
- In myasthenia gravis, antibody to acetylcholine receptors of skeletal muscle is formed which blocks the neuromuscular transmission at the motor end plate, resulting in muscle weakness.
- In male sterility, the anti-sperm antibody is formed which reacts with spermatozoa and causes impaired motility as well as cellular injury.
- In type 1 diabetes mellitus, islet cell autoantibodies are formed which react against islet cell tissue.
Question 6. Write in Short Type 3 hypersensitivity reaction.
Answer:
Type 3 hypersensitivity reaction is also known as an immune complex-mediated reaction or Arthus reaction.
Type 3 reactions result from the deposition of antigen—antibody complexes on tissues, which is followed by activation of the complement system and inflammatory reaction, resulting in cell injury. The onset of type 3 reaction takes place about 6 hours after exposure to the antigen.
Type 3 Hypersensitivity Etiology
There are 3 types of possible etiologic factors precipitating type 3 reaction:
1. Persistence of lowgrade microbial infection: A low-grade infection with bacteria or viruses stimulates a somewhat weak antibody response. The persistence of infection (antigen) and corresponding weak antibody response leads to chronic antigen-antibody complex formation. Since these complexes fail to get eliminated from body fluids, they are instead deposited in tissues, For Example. in blood vessel walls, glo- merely, joint tissue, etc.
2. Extrinsic environmental antigen: Exogenous antigens may be inhaled into the lungs, For Example. antigens derived from molds, plants, or animals. The inhaled antigen combines with antibodies in the alveolar fluid and forms antigen—antibody complex which is deposited in the alveolar walls.
3. Autoimmune process: Another sequence in type 3 reaction can be the formation of autoantibodies against their own tissue (self-antigen) forming an autoantibody-self antigen complex. Such self-antigens can be circulating (For Example. IgA) or tissue derived (For Example. DNA). Immune complexes containing both components from the body’s own system can thus be deposited in tissues.
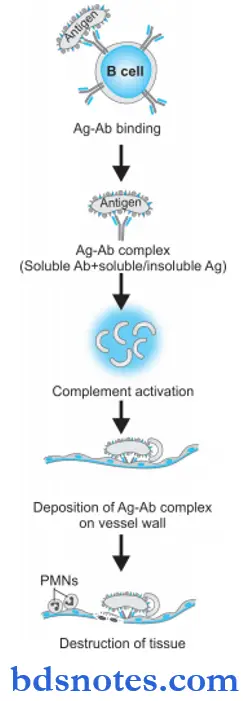
Type 3 hypersensitivity Pathogenesis
Type 3 reaction has participation by IgG and IgM antibodies, neutrophils, mast cells, and complement. The sequence of underlying mechanisms is as follows:
- Immune complexes are formed by the interaction of soluble antibodies and soluble or insoluble antigens.
- Immune complexes which fail to get removed from body fluid get deposited into tissues. Generally, small and intermediate-sized antibodies and antigens precipitate out of the body’s fluid and get deposited in tissues.
- Fc component of antibody links with complement and activates the classical pathway of complement resulting in the formation of C3a, C5a, and membrane attack complex.
- C3a stimulates the release of histamine from mast cells and its resultant effects of increased vascular permeability and edema.
- C5a releases proinflammatory mediators and chemotactic agents for neutrophils.
- Accumulated neutrophils and macrophages in the tissue release cytokines and result in tissue destruction.
Examples of Type 3 Reaction
Common examples of cell injury by type III injury are as under:
- Immune complex glomerulonephritis in which the antigen may be glomerular basement membrane or exogenous agents (For Example. Streptococcal antigen).
- Systemic lupus erythematosus in which there is a nuclear antigen (DNA, RNA) and there is the formation of antinuclear and anti-DNA autoantibodies.
- Rheumatoid arthritis in which there is a nuclear antigen.
- Farmer’s lung in which actinomycetes-contaminated hay acts as an antigen.
- Polyarteritis nodosa and Wegener’s granulomatosis with antineutrophil cytoplasmic antigen.
Question 7. Define antigenantibody reactions.
Answer:
Antigens and antibodies combine with each other specifically and in an observable manner. Antigen-antibody reactions in vitro are known as serological reactions.
Question 8. Discuss the chemical and physical structure of amyloidosis. What special stains are used to demonstrate amyloidosis?
Or
Write a brief on the staining characteristics of amyloid.
Or
Describe amyloidosis.
Answer:
Physical and Chemical Structure of Amyloidosis
It emerges that on the basis of morphology and physical characteristics, all forms of amyloid are similar in appearance but are chemically heterogeneous.
Amyloidosis Physical Structure
Amyloid consists of two types of proteins:
1. Fibrillar proteins: The majority of all forms of amyloid consist of a meshwork of fibrillar proteins. The girls are delicate random, dispersed, and non-branching, each measuring 7.5 to 10 nm in diameter and having an indefinite length. Each girl is further composed of the double helix of two pleated sheets in the form of twin filaments separated by a clear space. By X-ray crystallography and infra-red spectroscopy, fibrils are shown to have a cross-pleated sheet configuration which produces 1000 A° periodicity. These properties give amyloid its characteristic staining properties with Congo red dye and birefringence under polarising microscopy.
- AL protein: AL amyloid fibril protein is derived from the immunoglobulin light chain, which may be a complete light chain, or may include an amino-terminal segment and part of the C region of the immunoglobulin light chain. AL fibril protein is more frequently derived from the lambda light chain (twice more common) than kappa. AL-type official protein is produced by immunoglobulin-secreting cells and is therefore seen in association with plasma cell dyscrasias and is included in primary systemic amyloidosis.
- AA protein: AA fibril protein is composed of protein with a molecular weight of 8.5 kDa which is derived from a larger precursor protein in the serum called serum amyloid-associated protein with a molecular weight of 12.5 kDA. Deposits of AA amyloid do not have sequence homology. In the plasma, serum amyloid-associated protein circulates in association with HDL3 (high-density lipoprotein). Serum amyloid-associated protein is an acute phase reactant protein synthesized in the liver, in response to chronic inflammatory and traumatic conditions and thus the level of serum amyloid-associated protein is high in these conditions. AA fibril protein is found in secondary amyloidosis which is seen in association with several examples of chronic infectious and autoimmune inflammatory diseases and disseminated malignancies.
2. Nonfirillar components: They consist of 5% of amyloid material. They are:
- Amyloid P: It is synthesized from the liver and is structurally related to C reactive protein. On the basis of electron microscopy, it has a pentagonal profile or doughnut shape with an external diameter of 9 nm and an internal diameter of 4 nm.
- Apolipoprotein E: It is a regulator of lipoprotein metabolism and is found in all types of amyloid
- Sulfated glycosaminoglycans: They are constituents of matrix proteins, For Example. heparin sulfate
- Protein X: It is seen in cases of promises.
Amyloidosis Chemical Structure
Chemical analysis of fibril proteins of amyloid reveals the heterogeneous nature of amyloid. The three major forms of amyloid protein are:
1. AL protein, i.e. amyloid light protein: It is derived from lambda light chain and then kappa. AL type of viral protein is produced by immunoglobulin-secreting cells and is seen in plasma cell dyscrasias.
2. AA protein, i.e. amyloid associated protein: It is derived from a larger precursor protein in the serum called SAA. Deposits of AA amyloid don’t have sequence homology. It is found in secondary amyloidosis.
3. Other proteins:
- Transthyretin, i.e. TTR: It is a serum protein synthesized in the liver and normally transports thyroxine and retinol. Single amino acid substitution mutations in the structure of TTR results in a variant form of protein that is responsible for this form of amyloidosis termed ATTR. ATTR is the most common form of here do familial amyloidosis seen in familial amyloid
polyneuropathies. - A β2 microglobulin, i.e. A β2M: This form of amyloid is seen in cases of long-term hemodialysis (for 10-12 years). It is a small protein that is a normal component of major histocompatibility complex (MHC) class 1 and has a β-pleated sheet structure. β2M is an 11.8 kDa protein that is not filtered by the hemodialysis membrane and thus there is a high serum concentration of β2M protein in these patients. Although the deposit due to Aβ2M may be systemic in distribution, it has a predilection for bones and joints.
- Beta-amyloid protein (Aβ): Aβ is distinct from Aβ2M and is deposited in cerebral amyloid angiopathy and neurofibrillary tangles in Alzheimer’s disease. Aβ is derived from amyloid beta precursor protein (AβPP). The latter is a cell surface protein having a single transmembranous domain that functions as a receptor. The Aβ portion of this protein is seen extending into the extracellular region. Out of three intramembranous cleavage sites secretase—α, β, and γ, partial proteolysis of AβPP due to cleavage of β-secretase and γ-secretase sites generates Aβ, i.e. amyloidogenic protein in Alzheimer’s disease.
- IgG heavy chain amyloid (AH): It is derived from a truncated heavy chain of immunoglobulin.
- Amyloid from hormone precursor protein: These are procalcitonin, islet amyloid polypeptide, pro-insulin, and prolactin.
- Amyloid of prion protein(APrP): It is derived from precursor prion protein which is a plasma membrane glycoprotein. Prion proteins are proteinaceous infectious particles lacking in RNA or DNA. Amyloid in prognosis occurs due to an abnormally folded isoform of the PrP
- Miscellaneous here do familial forms of amyloid: It includes a variety of amyloid proteins reported recently.
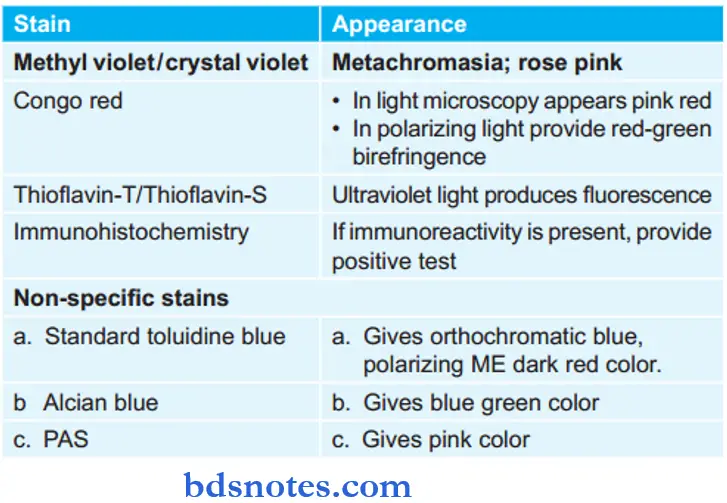
Special Stains Used to Demonstrate Amyloidosis
- H and E stain: Amyloid by light microscopy with H and E stain appears as extracellular, structureless, and eosinophilic hyaline material. It appears pink in color.
- Metachromatic stains: The metachromatic stains are methyl violet/crystal violet which imparts rose pink color to amyloid deposit.
- Congo red stain: Under light microscopy while staining the amyloid with Congo red stain it gives pink red color. In polarized light, it provides re-green birefringence.
- Thioflavin-T and S: Under this amyloid fluoresces yellow color.
- Immunohistochemistry: Various antibody stains against specific antigenic protein types of amyloid are available. The most common antibodies are anti-AA, anti-lambda, and anti-kappa antibodies to differentiate between different types of amyloids.
- Nonspecifi Stains:
- Standard Toluidine Blue: It gives orthochromatic blue color to amyloid which under polarizing microscopy produces dark red birefringence.
- Alcian Blue: Impart blue-green color and is used for mucopolysaccharide content in amyloid.
- Periodic Schifftain (PAS): Used for demonstration of carbohydrate content. It appears pink in color.
Question 9. Write a short note on primary amyloidosis.
Or
Write a short note on AL amyloid.
Answer:
Primary amyloidosis consists of AL fibril protein which is derived from the lambda chain and more common from the kappa chain. Primary amyloidosis is more common in developed countries.
Associated disease: Plasma cell dyscrasias, For Example. multiple myeloma, B-cell lymphoma, heavy chain disease, solitary plasmacytoma.
Primary amyloidosis Pathogenesis
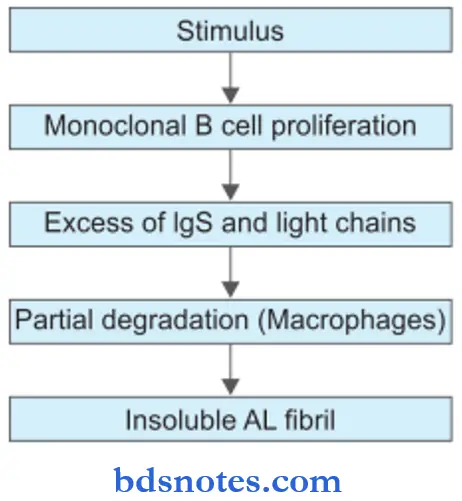
- The stimulus for the production of AL amyloid is some disorder of immunoglobulin synthesis, For Example. multiple myeloma and B cell lymphoma.
- Excessive immunoglobulin production is present in the form of monoclonal gammopathy. This takes place by monoclonal proliferation of plasma cells, B lymphocytes, and their precursors.
- There is partial degradation in the form of limited proteolysis of larger protein molecules in macrophages which are associated with AL protein.
- Non-fibrillar components like AP and glycosaminoglycans play some role in the folding and aggregation of viral proteins which are insoluble.
- Organ distribution: Kidney, heart, bowel, nerve, skin, respiratory tract, and skeletal muscles.
Investigations for Primary Amyloidosis
- Serum/urine electrophoresis
- Immunoelectrophoresis
- Bone marrow aspiration
- Specific immunoassays with anti-kappa antibodies.
primary amyloidosis Treatment
Treatment for primary amyloidosis is targeted at reducing the underlying clonal expansion of plasma cells.
Question 10. Write a short note on Amyloidosis.
Or
Write a short answer on amyloidosis.
Answer:
Amyloidosis is the term used for a group of diseases characterized by the extracellular deposition of a fibrillar proteinaceous substance called amyloid.
Classification of Amyloidosis
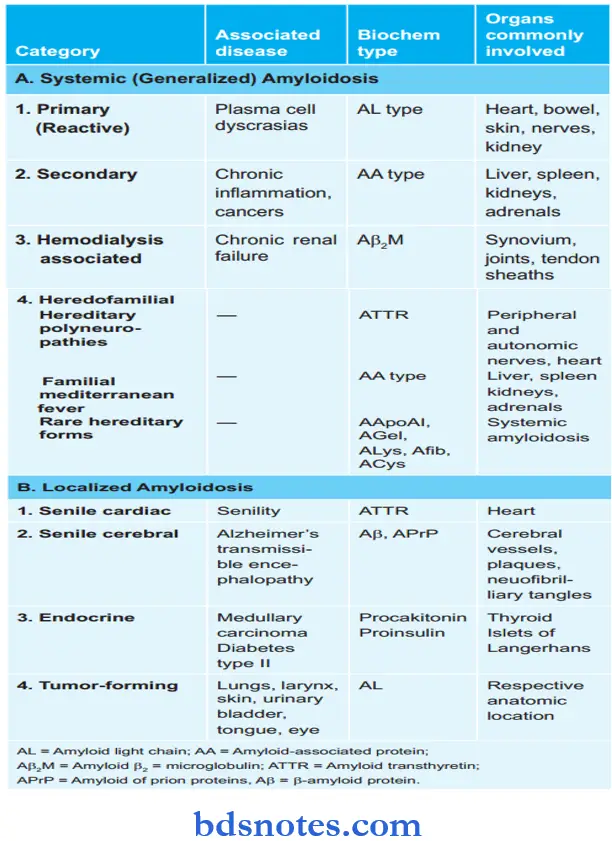
Primary Amyloidosis
- Biochemical Composition: AL protein
- Associated Organ: Kidney, heart, bowel, nerve, and skin
- Associated disease: Plasma cell dyscrasias, multiple myeloma, B-cell lymphoma.
Amyloidosis Pathogenesis
Morphology of Amyloidosis
- Primary amyloidosis: This affects the kidney, liver, spleen, lymph node, adrenal gland, and thyroid gland
- Secondary amyloidosis: This affects the heart, kidney, GIT, peripheral nerves, skin, and tongue.
Amyloidosis Gross Features
- Affected organs are fim, enlarged, and waxy.
- Painting the cut surface with iodine imparts yellow color which changes to bluish violet after the application of sulphuric acid.
Pathologic Changes in Amyloidosis of Organ
- The affected organ is usually enlarged, pale, and rubbery cut surface shows fim, waxy and translucent parenchyma.
- The deposits of amyloid are found in extracellular locations, initially in the walls of small blood vessels producing microscopic changes and effects.
Question 11. Write the routes of transmission of the human immunodeficiency virus.
Or
Write short notes on routes of spread of HIV infection.
Answer:
Transmission of HIV infection occurs by following routes:
- Sexual transmission: Sexual contact is the main mode of spread and constitutes 75% of all cases of HIV transmission. Most cases of AIDS in the industrialized world such occur in homosexual or bisexual males while heterosexual promiscuity seems to be the dominant mode of HIV infection. Other sexually transmitted diseases (STDs) may act as cofactors for the spread of HIV in particular gonorrheal and chlamydial infection. Transmission from male to male and male to female is a more potent route than that from female to male.
- Transmission via blood and blood products: This mode of transmission is the next largest group (25%) and occurs in 3 types of high-risk populations:
- Intravenous drug abusers by sharing needles, syringes, etc. comprise a large group.
- Hemophiliacs who have received large amounts of clotting factor concentrates from pooled blood components from multiple donors.
- Recipients of HIV-infected blood and blood products who have received multiple transfusions of whole blood or components like platelets and plasma.
- Perinatal transmission: HIV infection occurs from the infected mother to the newborn during pregnancy transplacentally or in the immediate postpartum period through contamination with maternal blood, infected amniotic fluid, and breast milk.
- Occupational transmission: There have been a small number of healthcare workers, laboratory workers, and those engaged in the disposal of waste of sharps who have developed HIV infection by occupational exposure to HIV-infected material, most often by needle-stick injury. It is imperative that these workers follow the CDC guidelines for universal precautions which include disinfecting and sterilizing all reusable devices and use of bleaching solution for disinfecting all blood spillage.
- Transmission by other body fluids: Besides blood, HIV has been isolated and identified from a number of body fluids such as saliva, tears, sweat, urine, semen, vaginal secretions, cervical secretions, breast milk, CSF, synovial, pleural, peritoneal and pericardial fluid, there is no definite evidence that HIV transmission can occur by any of these flids; isolated cases of such infection reported are likely due to concomitant contamination with HIV-infected blood.
Question 12. Write In Brief About Routes Of Transmission Of Hiv In Relation To Dentistry.
Answer:
The routes of transmission of HIV in relation to dentistry are as follows:
- By using contaminated needles in the mucous membrane of the mouth.
- By using unsterilized partial dentures and fixed dentures.
- By using unsterilized dental instruments.
- By transfusion of contaminated blood.
Question 13. Mention briefly methods used in viral diagnosis.
Answer:
The various methods used are as follows:
1. Microscopic examination: Ordinary microscope for detection of the inclusion body is useful. The ordinary microscope is also useful for the examination of stained smears of tissue sections for characteristic histological changes. Immunofluorescent techniques are used for the rapid identification of viruses.
2. Demonstration of virus antigen: In smallpox, the antigen is abundant in lesions and so used to be demonstrated rapidly by precipitation in gel (PIG) or immunofluorescent technique.
3. Isolation of virus: The methods of isolation of different viruses are different. Viruses are identified by disease and the particular lesions they produce. Tissue culture is being more popular as it is convenient and cheap.
4. Serological diagnosis: Rising titer of antibodies to the virus during disease is supporting and almost confirmatory evidence. The serological methods widely used are complement fixation, hemagglutination inhibition, neutralization, immunology essence, radioimmunoassay (RIA), immunoelectrophoresis, ELISA, etc.
Question 14. Write a short note on autoimmune disease.
Answer:
Autoimmunity is a state in which the body’s immune system fails to distinguish between ‘self’ and ‘non-self and reacts by the formation of autoantibodies against one’s own tissue antigens.
Normally, the immune system of the body is able to distinguish self from non-self antigens by the following mechanisms:
- Clonal elimination: According to this theory during embryonic development, T cells maturing in the thymus acquire the ability to distinguish self from non-self. These T cells are then eliminated by apoptosis for the tolerant individual.
- Concept of clonal energy: According to this mechanism, T lymphocytes that have acquired the ability to distinguish self from non-self are not eliminated but instead become non-responsive and inactive.
- Suppressor T cells: According to this mechanism, the tolerance is achieved by a population of specific suppressor T cells which do not allow the antigen-responsive cells to proliferate and differentiate.
Autoimmune disease Pathogenesis
- Immunological factors: Failure of immunological mechanisms of tolerance initiates autoimmunity. These mechanisms are as follows:
- Polyclonal activation of B cells: B cells may be directly activated by stimuli such as infection with microorganisms and their products leading to bypassing of T cell tolerance.
- Generation of self-reacting B cell clones may also lead to bypassing of T cell tolerance.
- Decreased T suppressor and increased T helper cell activity. Loss of T suppressor cells and an increase in T helper cell activities may lead to high levels of auto-antibody production by B cells contributing to autoimmunity.
- Fluctuation of immunological network control may cause failure of mechanisms of immune tolerance.
- Sequestered antigen released from tissues: ‘Selfantigen’ which is completely sequestered may act as ‘foreign-antigen’ if introduced into the circulation later.
- Genetic factors: There is evidence in support of genetic factors in the pathogenesis of autoimmunity as under:
- There is increased expression of Class II HLA antigens on tissues involved in autoimmunity
- There is an increased familial incidence of some autoimmune disorders.
- Microbial factors: Infection with microorganisms, particularly viruses (For Example. EBV infection), and less often bacteria (For Example. streptococci, Klebsiella) and M mycoplasma have been implicated in the pathogenesis of autoimmune diseases.
Types of Autoimmune Diseases
Depending upon the type of autoantibody formation, autoimmune diseases are broadly classified into two groups:
- Organ-specific diseases: In these, the autoantibodies formed react specifically against an organ or target tissue component and cause its chronic inflammatory destruction. The tissues affected are endocrine glands, alimentary tract, blood cells, and various other tissues and organizers:
- Nonorgan specific diseases: These are diseases in which a number of autoantibodies are formed which react with antigens in many tissues and thus cause systemic lesions. Examples of this group are various systemic collagen diseases.
Question 15. Write a note on laboratory diagnosis of amyloidosis.
Answer:
Laboratory Diagnosis of Amyloidosis
- Biopsy examination: Histologic examination of biopsy material is the commonest and confirmatory method for diagnosis in a suspected case of amyloidosis. Biopsy of an obviously affected organ is likely to offer the best results, For Example. kidney biopsy in a case on dialysis, sural nerve biopsy in familial polyneuropathy. The affected organ reveals deposits of amyloid in the walls of blood vessels.
- In vivo Congo red test: A known quantity of Congo red dye may be injected intravenously into a living patient. If amyloidosis is present, the dye gets bound to amyloid deposits and its levels in the blood rapidly decline. The test is, however, not popular due to the risk of anaphylaxis to the injected dye.
- Other tests: A few other tests which are not diagnostic but are supportive of amyloid disease are protein electrophoresis, immunoelectrophoresis of urine and serum, and bone marrow aspiration.
- Special stains used to demonstrate amyloidosis
- H&E stain: Amyloid by light microscopy with H and E stain appears as extracellular, structure, and eosinophilic hyaline material. It appears pink in color.
- Metachromatic stains: The metachromatic stains are methyl violet/crystal violet which imparts rose pink color to amyloid deposit.
- Congo red stain: Underlightmicroscopywhilestaining the amyloid with Congo red stain it gives pink red color. In polarizing light, it provides red-green birefringence.
- ThioflavinT and S: Under this amyloid emits secondary fluoresces yellow color.
- Immunohistochemistry: Various antibody stains against specific antigenic protein types of amyloid are available. The most common antibodies are anti-AA, anti-lambda, and anti-kappa antibodies to differentiate between different types of amyloids.
- Nonspecifi stains:
- Standard Toluidine Blue: It gives orthochromatic blue color to amyloid which under polarizing microscopy produces dark red birefringence.
- Alcian blue: Impart blue-green color and is used for mucopolysaccharide content in amyloid.
- Periodic Schifftain (PAS): Used for demonstration of carbohydrate content. It appears pink in color.
Question 16. Describe etiopathogenesis
Or
Describe etiopathogenesis and classify amyloidosis.
Answer:
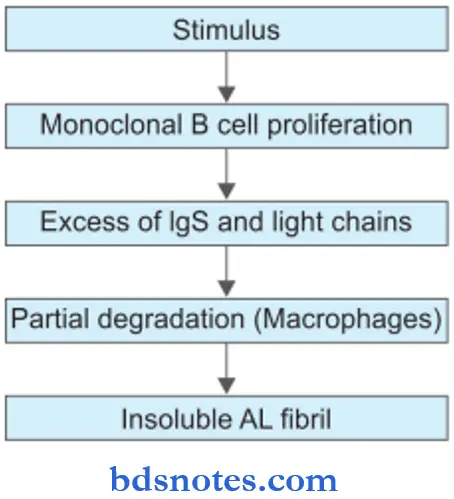
Etiopathogenesis of Amyloidosis
Different mechanisms are involved in different types of amyloid. In general amyloidogenesis in vivo occurs in the following sequence:
- A pool of amyloidogenic precursor protein is present in the circulation in different clinical settings and in response to stimuli, For Example. increased hepatic synthesis of AA or ATTR, increased synthesis of AL, etc.
- Nidus for fibrillogenesis: Thereby an alteration in the microenvironment to stimulate the deposition of amyloid protein. This alteration involves changes and interactions between basement membrane proteins and amyloidogenic proteins.
- Partial degradation or proteolysis: It occurs prior to the deposition of fibrillar protein which may occur in macrophages or reticuloendothelial cells, For Example. inAL, AA, etc. Exceptions to this generalization are seen in ATTR, Ab2M, and prognosis.
- The role of non-fibrillar components such as AP, apoE, and GAGs in amyloidosis is unclear; probably they facilitate the aggregation of proteins and protein folding leading to fibril formation, substrate adhesion, and protection from degradation.
Deposition of AL Amyloid
- The stimulus for the production of AL amyloid is some disorder of immunoglobin synthesis, For Example. multiple myeloma, B cell lymphoma, and other plasma cell dyscrasias.
- Excessive immunoglobulin production is in the form of monoclonal gammopathy, i.e. there is the production of either intact immunoglobulin, or λ light chain, or κ light chain, or rarely heavy chains. This takes place through monoclonal
the proliferation of plasma cells, B lymphocytes, or their precursors. - Partial degradation in the form of limited proteolysis of larger protein molecules occurs in macrophages which are anatomically closely associated with AL amyloid.
- Non-fibrillar components like AP and GAGs play some role in the folding and aggregation of viral proteins.
Deposition of AA Amyloid
- AA amyloid is directly related to SAA levels. SAA is a high-density lipoprotein the levels of which are elevated in long-standing tissue destruction accompanied by chronic inflammation.
- SAA is synthesized by the liver in response to cytokines, notably interleukin l and 6, from activated macrophages. However, SAA levels in isolation do not always lead to AA amyloid.
- As in AL amyloid, partial degradation in the form of limited proteolysis takes place in reticuloendothelial cells.
- In AA amyloid, a significant role is played by glycoprotein, amyloid enhancing factor (AEF). The composition of AEF is not known. It is elaborated in chronic inflammation, cancer, and familial Mediterranean fever. Possibly AEF acts as a nidus for the deposition of fibrils in AA amyloid.
- As in AL amyloid, there is the role of the AP component and glycosaminoglycans in the final protein aggregation and to protect it from disaggregation again.
Question 17. Define and classify hypersensitivity reactions. Discuss Type 4 (delayed hypersensitivity) in brief.
Or
Write a short note on type 4 hypersensitivity reaction.
Or
Write a brief on the delayed type of hypersensitivity.
Or
Write a note on the delayed type of hypersensitivity.
Answer:
Hypersensitivity is defined as a state of exaggerated immune response to an antigen.
Classification By Coomb and Gel (1963)
- Immediate Type
- Type 1: Anaphylactic, atopic reaction
- Type 2: Cytotoxic reaction
- Type 3: Immune complex reaction.
- Delayed Type
- Type 4: Cell-mediated reaction
- Later on, Type 5 hypersensitivity reaction was also described, i.e. stimulatory type.
Type 4 (Delayed Hypersensitivity)
Type 4 hypersensitivity reaction is also known as delayed hypersensitivity or T cell-mediated reaction.
- The reaction is mediated by sensitized T-lymphocytes which, on contact with a specific antigen, release lymphokines that cause biological effects on macrophages, leucocytes, and tissue cells.
- Type 4 or delayed type of hypersensitivity occurs within 48-72 hours of antigen challenge. As it is not antibody-mediated, it cannot be passively transferred by serum but can be transferred by lymphocytes or the transfer factor.
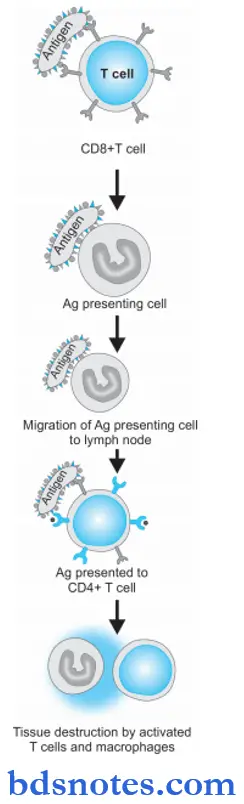
Type 4 hypersensitivity Pathogenesis
Type 4 reaction involves the role of mast cells and basophils, macrophages, and CD8+ T cells. Briefly, the mechanism of the type 4 reaction is as under:
- The antigen is recognized by CD8+ T cells (cytotoxic T cells) and is processed by antigen-presenting cells.
- Antigen-presenting cells migrate to lymph nodes where antigen is presented to helper T cells (CD4+ T cells).
- Helper T cells release cytokines that stimulate T cell proliferation and activate macrophages.
- ActivatedTcellsandmacrophagesreleaseproinflmmatory mediators and cause cell destruction.
Types of Delayed Hypersensitivity
Two types of delayed hypersensitivity reactions are well recognized, the tuberculin (infection) type and the contact dermatitis type.
Tuberculin (Infection) Type
- When a small dose of tuberculin is injected intradermally in an individual sensitized to tuberculoprotein by prior infection or immunization, an erythema and swelling (induration) occur at the site of injection within 48-72 hours.
- The injection site is infiltrated by a large number of lymphocytes and about 10-12% macrophages.
- In unsensitised individuals, the tuberculin injection provokes no response.
- Purified protein derivative (PPD) which is the active material of tubercle bacilli, is used in tuberculin tests.
- The tuberculin test is a useful indicator for delayed hypersensitivity to the bacillus.
- Cell-mediated hypersensitivity reaction develops in many infections with bacteria (M. tuberculosis, M. leprae), fungi, and parasites.
- It occurs when the infection is subacute or chronic and the pathogen is intracellular.
- Various skin tests are performed to detect delayed types of hypersensitivity.
- A positive skin test does not indicate a present infection but implies the person has been infected or immunized by microorganisms in the past. Some of these skin tests include:
- Lepromin test: It is positive in tuberculoid leprosy but negative in the lepromatous type of leprosy.
- Frei test: This test is positive in lymphogranuloma venereum (LGV).
- Histoplasmin test: It is positive in histoplasmosis.
Contact Dermatitis Type
- Delayed hypersensitivity may sometimes develop as a result of skin contact with a range of sensitizing materials metals such as nickel and chromium, drugs such as penicillin or other antibiotics in ointments, simple chemicals like hair dyes, picryl chloride, dinitrochlorobenzene, cosmetics, and soaps.
- These substances can act as haptens.
- After absorption through the skin, these molecules combine with skin protein to become antigenic.
- Cell-mediated immunity is induced in the skin.
- As most of the antigens involved are fat soluble, their likely portal of entry is along the sebaceous glands.
- Sensitization is particularly liable to occur when the chemical is applied in an oily base (ointment or cream) on an inflamed area of the skin.
- The Langerhans cells of the skin carry these antigens to regional lymph nodes where T-lymphocytes are sensitized.
- On subsequent exposure to the offending agent, sensitized lymphocytes release lymphokines which cause superficial inflammation of the skin characterized by redness, induration, and vesiculation within 24-48 hours.
- The dermis is infiltrated predominantly by lymphocytes and a few macrophages.
Morphology of Classic Delayed Hypersensitivity
- There is an accumulation of mononuclear cells around small veins and venules producing perivascular cuffing.
- Presence of increased microvascular permeability
- Escape of plasma proteins leading to dermal edema or deposition of fibrin in the interstitium (induration)
- Fully developed lesions show endothelial hypertrophy and hyperplasia.
- There are persistent/non-degradable antigens that induce perivascular lymphocytic infiltrate replaced by macrophages in 2 to 3 weeks. Macrophages are converted into epithelioid cells, which aggregate to form granulomas.
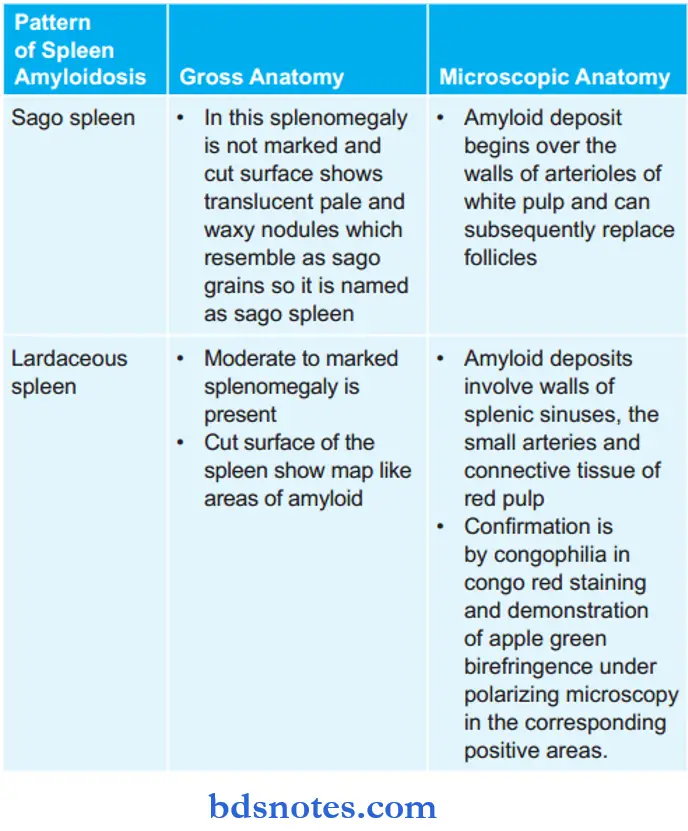
Question 18. Write a short note on the lardaceous spleen.
Answer:
It is the pattern of the spleen that is seen due to amyloid deposition inside the spleen.
Lardaceous spleen Gross Features
- There is generally moderate to marked splenomegaly.
- The cut surface of the spleen shows map-like areas of amyloid.
lardaceous spleen Microscopic Features
- Deposits involve the red pulp in the wall of splenic sinuses, in small arteries, and in the connective tissue.
- Confirmation is by zoophilia in congo red staining and demonstration of apple green birefringence under polarizing microscopy in the corresponding positive areas.
Question 19. Write a short note on AIDS.
Or
Write in brief on AIDS.
Or
Write briefly on acquired immunodeficiency syndrome (AIDS).
Answer:
AIDS means acquired immunodeficiency syndrome. AIDS is caused by HIV. HIV occurs in two main types HIV-1 and HIV-2.
AIDS Spread
- People who have unprotected vaginal or anal sex.
- People who have sex with many partners, thereby increase the chance that they will encounter a partner who is HIV infected.
- People who share needles for example for intravenous drug use, tattooing, or body piercing
- Babies of mothers who are HIV infected.
- People who have another STD, especially STDs that cause open sores or ulcers such as herpes, chancroid, or syphilis.
- Hemophiliacs and other people who frequently receive blood products (this risk is now very much diminished, but there are still countries where blood is not adequately screened).
- Health care workers, where precautions are neglected or failed for example through not wearing gloves or accidental needle stick injuries.
Question 20. Define amyloidosis. Enumerate special stains for amyloid. Describe the gross and microscopic anatomy of the spleen in amyloidosis.
Answer:
Amyloidosis is the term used for a group of diseases characterized by the extracellular deposition of a fibrillar proteinaceous substance called amyloid having a common morphological appearance, staining properties, and physical structure but with variable protein composition.
Enumeration of Special Stains for Amyloid
Gross and Microscopic Anatomy of Spleen in Amyloidosis
Amyloidosis in the spleen shows two patterns, i.e. sago spleen and lardaceous spleen.
Question 21. Define and enumerate types of hypersensitivity reactions.
Answer:
Hypersensitivity refers to a condition in which the immune response results in excessive reactions which lead to tissue damage, disease, or even death in the sensitized host.
Types of Hypersensitivity Reactions
Hypersensitivity reactions are classified into four major types by Coomb and Gel (1963).
Type 1: Hypersensitivity
Type 2: Cytotoxic
Type 3: Immune complex
Type 4: Delayed or cell-mediated
Type 1, 2, and 3 depend on the interaction of antigen with humoral antibodies and are known as immediate type reactions while Type IV is mediated by T-lymphocytes and is known as delayed hypersensitivity.
Later on, Type V hypersensitivity reaction was also described i.e. stimulatory type.

Leave a Reply