Hemodynamic Changes
Question 1. Write a short note on the mechanism of edema.
Or
Write in short on the pathophysiology of edema.
Answer:
The word edema means swelling.
“Edema may be defined as abnormal and excessive accumulation of fluid in interstitial tissue spaces and serous cavities”.
Mechanism of Edema
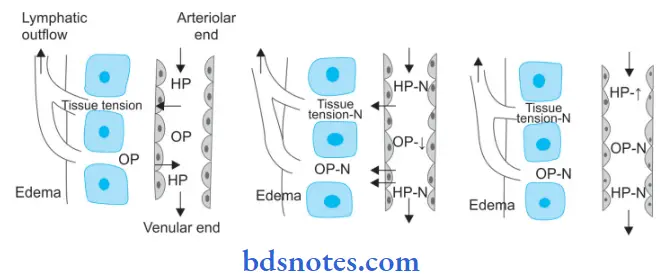
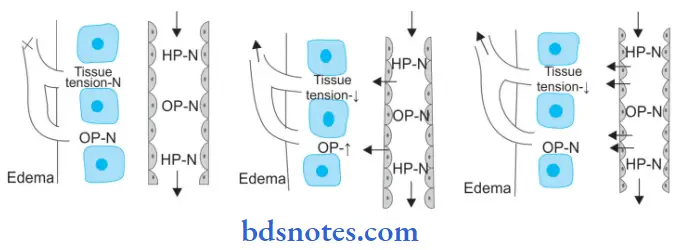
Read And Learn More: Pathology Question And Answers
The following six mechanisms may be operating singly or in combination to produce edema:
1. Decreased plasma oncotic pressure: Plasma oncotic pressure is exerted by the total amount of plasma proteins that tend to draw fluid into the vessels normally. A fall in the total plasma protein level (hypoproteinemia of less than 5 g/dl, mainly hypoalbuminemia), results in a lowering of plasma oncotic pressure in a way that it can no longer counteract the the effect of the hydrostatic pressure of blood. This results in the increased outward movement of fluid from the capillary wall and decreased inward movement of fluid from the interstitial space causing edema. Hypoproteinemia usually produces generalized edema. Out of the various plasma proteins, albumin has four times higher plasma oncotic pressure than globulin; thus it is mainly hypoalbuminemia (albumin below 2.5 g/dl) that generally results in edema.
2. Increased capillary hydrostatic pressure: The hydrostatic pressure of the capillary is the force that normally tends to drive fluid through the capillary wall into the interstitial space by counteracting the force of plasma oncotic pressure. A rise in the hydrostatic pressure at the venular end of the capillary which is normally low to a level more than the plasma oncotic pressure results in minimal or no reabsorption of fluid at the venular end, consequently leading to edema.
3. Lymphatic obstruction: Normally, the interstitial fluid in the tissue spaces escapes by way of lymphatics. Obstruction to the outflow of these channels causes localized edema, known as lymphedema.
4. Tissue factors: The two forces acting in the interstitial space—oncotic pressure of the interstitial space and tissue tension, are normally quite small and insignificant to counteract the effects of plasma oncotic pressure and capillary hydrostatic pressure respectively. However, in some situations, the tissue factors in combination with other mechanisms play a role in the causation of edema.
5. Increased capillary permeability: An intact capillary endothelium is a semipermeable membrane that permits the free flow of water and crystalloids but allows minimal passage of plasma proteins normally. However, when the capillary endothelium is injured by various ‘capillary poisons’ such as toxins and their products (For Example. histamine, anoxia, venoms, certain drugs, and chemicals), the capillary permeability to plasma proteins is enhanced due to the development of gaps between the endothelial cells causing leakage of plasma proteins into the interstitial fluid. This, in turn, causes reduced plasma oncotic pressure and elevated oncotic pressure of the interstitial fluid, consequently producing edema.
6. Sodium and water retention: The mechanism of edema by sodium and water retention in the extravascular compartment is best described in relation to derangement in the normal regulatory mechanism of sodium and water balance. Normally about 80% of tubules are under the influence of either intrinsic renal mechanism or extra-renal mechanism while retention of water is affected by the release of antidiuretic hormone. The possible factors responsible for causing edema by excessive retention of sodium and water in the extravascular compartment via stimulation of intrinsic renal and extrarenal mechanism as well as via release of ADH are:
- Reduced glomerular filtration rate in response to hypovolemia.
- Enhanced tubular reabsorption of sodium and consequently decreased renal excretion.
- Increased filtration factor, i.e. increased filtration of plasma from the glomerulus.
- Decreased capillary hydrostatic pressure is associated with increased renal vascular resistance.
Question 2. Give the difference between exudate and transudate.
Or
Write the differences between exudate and transudate.
Answer:
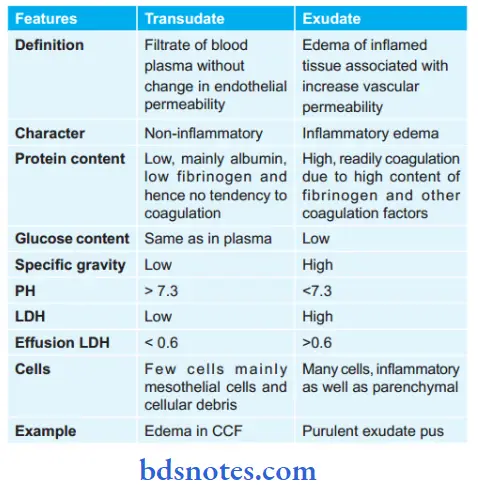
Question 3. What are circulatory disturbances (only name them)? Write changes in lung and liver due to chronic passive congestion.
Answer:
Circulatory or hemodynamic disturbances are considered under two broad headings:
- Disturbances in the volume of circulating blood:
- Hyperemia and congestion
- Hemorrhage and shock
- Disturbances due to obstruction:
Thrombus, embolism, ischemia and infarction.
1. Changes in the lung due to chronic venous congestion or CVC Lung: CVC of the lung occurs in left heart failure resulting in an increase in pulmonary venous pressure
Grossly
- Lungs become heavy and firm in consistency.
- The Cut surface is dark and rusty brown in color referred to as the brown induration of the lungs.
Histological features
- The alveolar septa is widened and thickened. Widen due to the presence of interstitial edema, dilated and congested capillaries. Thickened due to an increase in fibrous connective tissue.
- Rupture of the dilated and congested capillary may result in minute intra-alveolar hemorrhage
- Breakdown of RBCs liberates hemosiderin pigment which is taken up by alveolar macrophages so-called heart failure cells, present in the alveolar lamina.
- Brown induration is due to pigmentation and fibrosis.
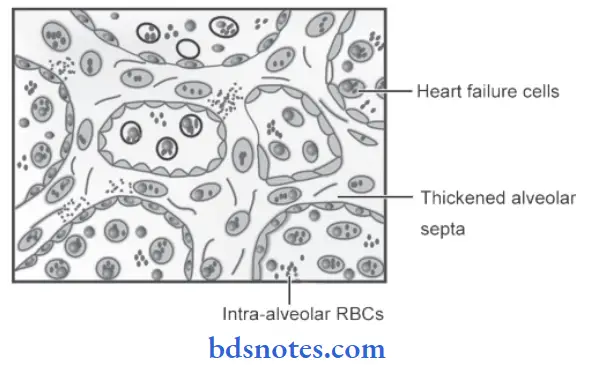
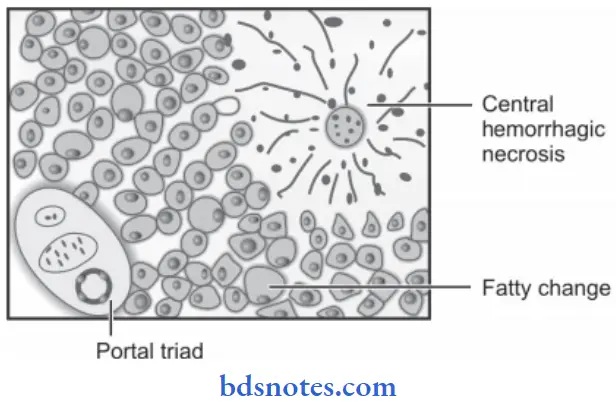
2. Changes in the liver due to chronic venous congestion or CVC Liver: Chronic venous congestion of the liver occurs in right heart failure and sometimes due to occlusion of the inferior vena cava and hepatic vein.
Grossly
- The liver is enlarged, and tender, and the capsule is tense.
- The cut surface shows nutmeg due to the red and yellow mottled appearance.
Microscopically
- Microscopically the changes in congestion are more marked in the centrilobular zone.
- The centrilobular hepatocytes undergo degenerative changes and eventually, centrilobular hemorrhage necrosis may be seen.
- Longstanding cases may show fie centrilobular fibrosis and regeneration of hepatocytes, resulting in cardiac cirrhosis.
- The peripheral zone of the lobule is less severely affected by chronic hypoxia and shows some fatty changes in hepatocytes.
Question 4. Write a brief on chronic venous congestion of the lung.
Answer:
CVC of the lung occurs in left heart failure resulting in an increase in pulmonary venous pressure.
lung Gross Features
- Lungs become heavy and firm in consistency.
- The Cut surface is dark and rusty brown in color which is referred to as the brown induration of the lungs.
Lung Microscopically
- The alveolar septa are widened and thickened. Widen due to the presence of interstitial edema, dilated and congested capillaries. Thickened due to an increase in fibrous connective tissue.
- Rupture of the dilated and congested capillary may result in minute intra-alveolar hemorrhage
- Breakdown of RBCs liberates hemosiderin pigment which is taken up by alveolar macrophages so-called heart failure cells, present in the alveolar lamina.
- Brown induration is due to pigmentation and fibrosis.
Question 5. Discuss hemorrhage and its effect.
Answer:
Hemorrhage is the escape of blood from blood vessels. The bleeding may occur externally or internally.
- The extravasation of blood into tissue with resultant swelling is known as “Hematoma”
- Large extravasation of blood into the skin and the mucous membrane is called as ecchymoses.
- A small area of hemorrhage is known as “petechiae”.
Cause of Hemorrhage
- Trauma
- Spontaneous hemorrhage: Septicemia, acute leukemias and bleeding diathesis
- Inflammatory lesion: Bleeding from chronic peptic ulcer, thyroid ulcer.
- Neoplastic invasions: Carcinoma of the tongue
- Vascular disease: Atherosclerosis
- Elevated pressure within vessels: Cerebral and retinal hemorrhage.
Effects of Hemorrhage
The effect of blood loss depends upon.
- Amount of blood loss
- Speed of blood loss
- Site of hemorrhage.
- Loss up to 20% of blood volume suddenly or slowly generally has little clinical effects because of compensatory mechanisms.
- A sudden loss of 33% of blood volume may cause death.
- While loss up to 50% of blood volume over a period of 24 hours may not be necessarily fatal.
- Chronic blood loss generally produces iron deficiency anemia.
- Acute hemorrhage may lead to hypovolemic shock.
Question 6. Write a short note about the classification of shock.
Answer:
Shock is defined as a clinical state of cardiovascular collapse characterized by an acute reduction of effective circulatory blood volume and inadequate perfusion of cells and tissues.
Classification of Shock
- Hypovolemic shock
- Cardiogenic shock
- Septic (Toxemic) shock
- Other types
- Traumatic shock
- Neurogenic shock
- Hypoadrenal shock
- Hypovolemic shock is due to a reduction in the blood volume. It is caused by severe hemorrhage and fluid loss.
- Cardiogenic shock is an acute circulatory failure with a sudden fall in cardiac output due to acute diseases of the heart without a reduction in blood volume. It is caused due to myocardial infarction, cardiac arrhythmias, and pulmonary embolism.
- Septic shock is due to bacterial infections which release toxins leading to shock. It is of two types based on the Gram staining of the organism, i.e. Gram-negative septicemia and Gram-positive septicemia.
- Traumatic shock results from trauma is due to hypovolemia but even after hemorrhage is controlled such patients continue to suffer the loss of plasma volume into the
interstitium of injured tissue. - Neurogenic shock: It results from the causes of interruption of sympathetic vasomotor supply.
- Hypoadrenal shock: It results from unknown adrenal insufficiency in which the patient fails to respond normally to the stress of trauma, surgery, or illness.
Question 7. Discuss etiopathology and complications of shock.
Or
Write in brief on the etiopathogenesis of shock.
Answer:
Shock: It is defined as a clinical state of cardiovascular collapse characterized by an acute reduction of effective circulatory blood volume and inadequate perfusion of cells and tissues.
Shock Etiopathology
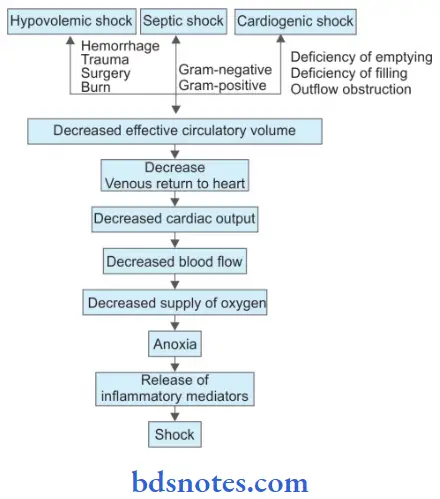
Complications of Shock
Life-threatening complications in shock are due to hypoxic cell injury which results in immune- inflammatory responses and activation of various cascades. Various complications of shock are:
- Acute respiratory distress syndrome
- Disseminated intravascular coagulation
- Acute renal failure
- Multiple organ dysfunction syndrome
With the progression of the condition, the patient can develop a stupor, comma, and finally death.
Question 8. Write a short note on hypovolemic shock.
Answer:
Reduction in blood volume leads to hypovolemic shock.
Hypovolemic shock Pathogenesis
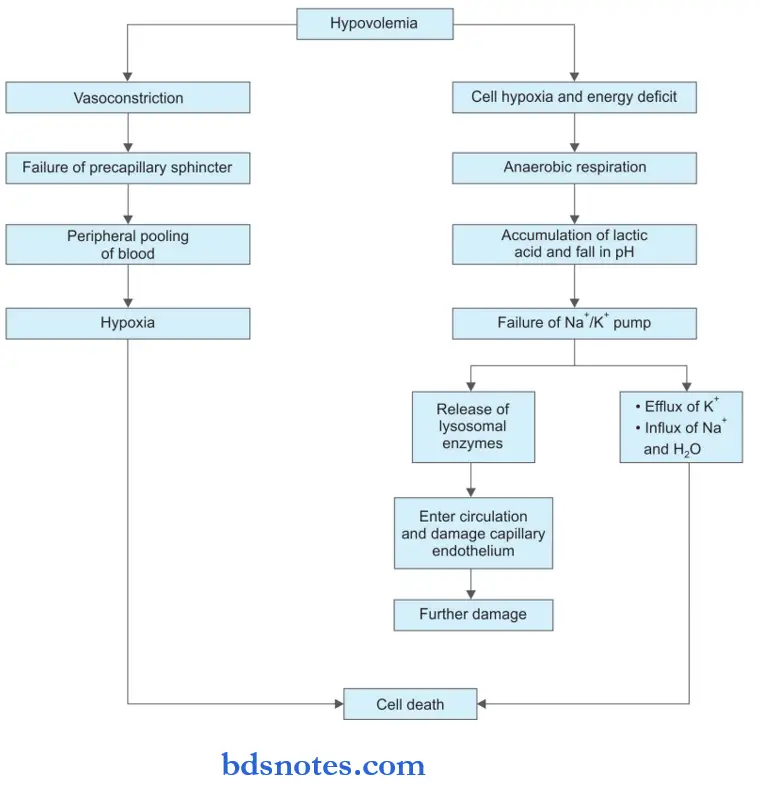
Hypovolemic shock Etiology
- Hemorrhage
- External from wounds, open fractures
- Internal from injury to the spleen, liver, mesentery or pelvis
- Severe burns, which result in loss of plasma fluid
- Vomiting and diarrhea of any cause lead to dehydration.
- Excessive use of diuretics
- Acute pancreatitis.
Question 9. Describe the etiopathogenesis of thrombosis.
Answer:
Human beings consist of a system in which blood remains in its fluid state during normal condition and this system also act as a guard to stop thrombosis and hemorrhage.
- The primary influence over the formation of a thrombus is known as Virchow’s triad. It consists of endothelial injury, altered blood flow and hypercoagulability of blood.
Thrombosis Endothelial Injury
- Blood vessel integrity is important for maintaining the normal flow of blood.
- Injurytobloodvesselleadstoexposureofthesubendothelial connective tissue which are thrombogenic and plays an important role in initiating hemostasis and thrombosis.
- An endothelial injury that leads to thrombogenesis occurs under diabetes mellitus, myocardial infarction, arterial diseases etc.
- Vascular injury causes loss of epithelium which exposes extracellular material, adhesion of the platelets, the release of tissue factors, and depletion of prostaglandins and plasminogen activators which leads to the formation of thrombus.
- Any of disturbance in the balance of prothrombotic and antithrombotic mechanisms of the endothelium of the vessel influences the local clotting mechanism.
- Endothelial dysfunction can lead to the release of more amount of more procoagulant factors which are plasminogen activator inhibitors, various tissue factors, etc.
Thrombosis Altered Blood Flow
- Normal axial flow of blood in it central stream consists of leucocytes and red blood cells. Platelets exist in a slow-moving laminar stream while which is adjacent to the central stream and the peripheral stream consists of slow-moving cell-free plasma close to the endothelial layer.
- Both turbulence and stasis occur in normal axial blood flow and disturb it.
- When the speed of flow of blood slows down blood cells containing platelets marginate toward the periphery and form a pavement close to the endothelium.
- As stasis leads to a higher release of oxygen from the blood, turbulence injures the endothelium which leads to the deposition of platelets and fibrin.
- Turbulence causes the formation of arterial and cardiac thrombi while stasis leads to the formation of venous thrombi.
Thrombosis Hypercoagulability of Blood
- Hypercoagulability is the alteration in the coagulation pathway that leads to thrombosis.
- It occur due to the following changes in the composition of blood:
- Increase in coagulation factors such as fibrinogen, prothrombin, and factor 7a, 8a and 10a.
- Increase in platelet count and its adhesiveness
- Decrease in levels of coagulation inhibitors i.e. antithrombin III, fibrin split products.
It is divided into two i.e. genetic and acquired factors Some of the genetic factors which predispose to hypercoagulability are:
- Deficiency of anti-thrombotic factors, i.e. anti-thrombin 3, protein C and S and defects in fibrinolysis.
- Increase in prothrombotic factors such as in factor V mutation, high level of factors 7, 9, 11, 8, Von Willebrand factor and fibrinogen.
Some of the acquired factors which predispose to hypercoagulability are:
- Venous stasis: It is due to prolonged immobilization and congestive cardiac failure.
- Increased platelet activation: In cancers, acute leukemias, myeloproliferative disorders, paroxysmal nocturnal hemoglobinuria, prosthetic cardiac valves, atrial fibrillation, etc.
- Due to increased hepatic synthesis of coagulation factors or reduced anticoagulant synthesis due to oral contraceptives and in pregnancy.
- Due to tissue injuries such as in surgery, fractures and extensive burns.
Question 10. Define thrombosis, its pathogenesis, and its complications.
Or
Write a short note on the complications of thrombosis.
Answer:
Thrombosis is defined as the process of formation of a solid mass in circulation from the constituents of flowing blood. The mass itself is known as a thrombus.
Thrombosis Complications
- Cardiac thrombi: Large thrombi in the heart may cause sudden death by mechanical obstruction of blood flow or thromboembolism to vital organizer:
- Arterial thrombi: These cause ischemic necrosis, infarct, and gangrene. Sudden death may occur following a thrombus of the coronary artery.
- Venous thrombi: They may cause the following effects.
- Thromboembolism
- Poor wound healing
- Skin ulcer
- Edema of the area drained
- Thrombophlebitis
- Painful white leg
- Capillary thrombi: Microthrombi in microvasculature give rise to disseminated intravascular coagulation.
Question 11. Describe factors responsible for thrombogenesis. Describe the morphology of the thrombus and its fate.
or
Write briefly on the possible fate of thrombi.
Or
Write a note on fate of the thrombus
Answer:
Factors Responsible for Thrombogenesis
There are three major contributors:
- Endothelial injury: It can be secondary to:
- Myocarditis
- Myocardial infarction
- Cardiac surgery
- Ulcerated atherosclerotic plaque
- Infected valve disease
- Prosthetic valves
- Radiation injury
- Chemical agents such as smoking, hypercholesterolemia, etc.
- Alteration in normal blood flow: It leads to
- Disruption of laminar flow
- Damage to endothelium
- Decreased hepatic clearance of activated coagulation factors
- Conditions predisposing to hypercoagulability It is divided into two i.e. genetic and acquired factors.
Some of the genetic factors which predispose to hypercoagulability are:
- Deficiency of anti-thrombotic factors, i.e. anti-thrombin 3, protein C and S, and defects in fibrinolysis.
- Increase in prothrombotic factors such as in factor 5 mutation, high level of factors 7, 9, 11, 8, Von Willebrand factor and fibrinogen.
Some of the acquired factors which predispose to hypercoagulability are:
- Venous stasis: It is due to prolonged immobilization and congestive cardiac failure.
- Increased platelet activation: In cancers, acute leukemias, myeloproliferative disorders, paroxysmal nocturnal hemoglobinuria, prosthetic cardiac valves, atrial fibrillation, etc.
- Due to increased hepatic synthesis of coagulation factors or reduced anticoagulant synthesis due to oral contraceptives and in pregnancy.
- Due to tissue injuries such as in surgery, fracture,s and extensive burns.
Morphology of Thrombus
- Cardiac thrombi: They develop in the area of turbulence and at the sites of endocardial injury. Thrombi which are present in cardiac chambers or aorta shows the presence of laminations or lines of Zahn, i.e. a pale layer of fibrin and platelets alternating with a dark layer of RBCs. Thrombi present in small arteries or veins do not show lines of Zahn.
- Mural thrombi: They are attached to a single wall of an underlying structure and is usually capacious lumina of heart chambers and blood vessels.
- Arterial thrombi: They are occlusive when they involve small vessels and large vessels. Arterial thrombi tend to be white.
- Venous thrombi: They are invariably occlusive and consist of a large RBC component, this is because they are formed in a static environment. They are also known as red or stasis thrombi. These thrombi always have a point of attachment to the underlying structure and are finest at the point of origin.
- Contraction of the thrombus provides a slit-like lumen that restores blood flow leading to the propagation of the thrombus upstream and downstream.
Fate of Thrombus
- Resolution: Thrombus activates the fibrinolytic system with the release of firing which may dissolve the thrombi completely resulting in the resolution. Usually, lysis is complete in small venous thrombi while large thrombi may not be dissolved. Fibrinolytic activity can be accentuated by the administration of thrombolytic substances, especially in the early stage when firing is in monomeric form.
- Organization: If the thrombus is not removed, it starts getting organized.
- Phagocytic cells phagocytosed firing and cell debris.
- The proteolytic enzymes start digesting the coagulum.
- Capillary grows in the thrombus from the site of its attachment and fibroblast starts invading the thrombus.
- Thrombus in this way becomes the part of vessel wall.
- The new vascular channels in it may re-establish the blood flow. It is called as recanalization.
- The fibrosis thrombus may undergo hyalinization and calcification.
Propagation: The thrombus may enlarge in size due to more deposition from constituents of flowing blood and obstructing some important vessels.
Thromboembolism: Thrombi in the early stage or infected thrombi are friable and may get detached from the vessel wall which produces ill effects at the site of their lodgement.
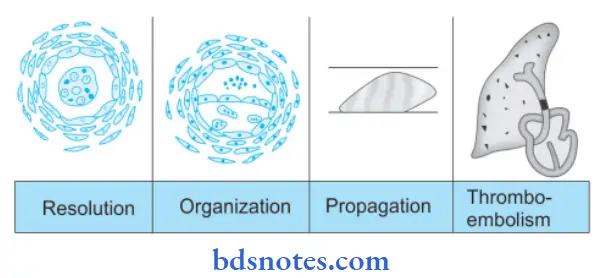
Question 12. How will you differentiate thrombosis and coagulation?
Answer:
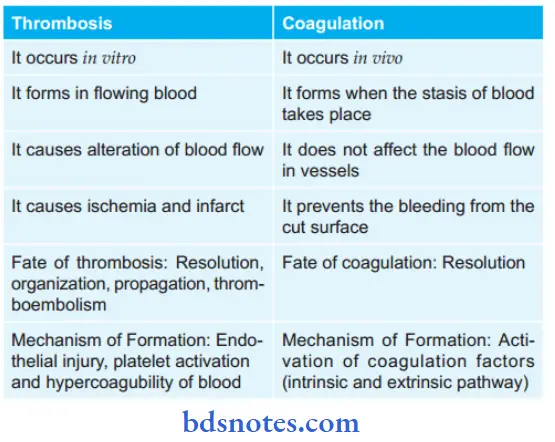
Question 13. Write a short note on infarct.
Answer:
Infarction is the process of tissue necrosis resulting from some of circulatory insufficiency, the localized area of necrosis so developed is called as an infarct.
Infarct Etiology
- Most commonly infarcts are caused by interrupted arterial blood supply called ischemic necrosis.
- Less commonly venous obstruction can produce infarct termed as stagnant hypoxia.
- Generally, sudden complete and continuous occlusion by thrombosis or emboli produces infarcts.
- Infarcts may be produced by non-occlusive circulatory insufficiency.
Types of Infarcts
Infarcts are classifid depending upon diffrent features:
- According to their color:
- Pale or anemic, due to arterial occlusion and are seen in compact organs, For Example. in the kidneys, heart, and spleen.
- Red or hemorrhagic, seen in soft loose tissues and are caused either by pulmonary arterial obstruction (For Example. in the lungs) or by arterial or venous occlusion ( For Example. in the intestines).
- According to their age:
- Recent or fresh
- Old or healed
- According to the presence or absence of infection:
- Bland, when free of bacterial contamination
- Septic, when infected.
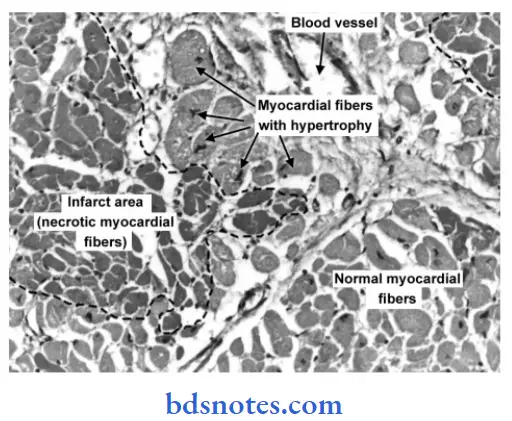
Infarct Pathogenesis
The process of infarction takes place as follows:
- Localized hyperemia due to local anoxemia occurs immediately after obstruction of the blood supply.
- Within a few hours, the affected part becomes swollen due to edema and hemorrhage. The amount of hemorrhage is variable, being more marked in the lungs and spleen, and less extensive in the kidneys and heart.
- Cellular changes such as cloudy swelling and degeneration appear early (reversible cell injury), while cell death (irreversible cell injury or necrosis) occurs in 12-48 hours.
- There is progressive proteolysis of the necrotic tissue and there is lysis of the red cells.
- An acute inflammatory reaction and hyperemia appear at the same time in the surrounding tissues in response to products of proteolysis.
- Blood pigments, hematoid and hemosiderin, liberated by the lysis of RBCs are deposited in the infarct. At this stage, most infarcts become pale grey due to the loss of red cells.
- Following this, there is a progressive in the growth of granulation tissue from the margin of the infarct so that eventually the infarct is replaced by a fibrous scar. Dystrophic calcification may occur sometimes.
Morphologic Features
Some general morphological features of infarcts are:
Gross Features
- Infarcts of solid organs are usually wedge-shaped, the apex pointing towards the occluded artery and the wide base on the surface of the organ.
- Infarcts due to arterial occlusion are generally pale while those due to venous obstruction are hemorrhagic.
- Most infarcts become pale later as the red cells are lysed but pulmonary infarcts never become pale due to the extensive amount of blood.
- Cerebral infarcts are poorly defined with central softening (encephalomalacia).
- Recent infarcts are generally slightly elevated over the surface while the old infarcts are shrunken and depressed under the surface of the organ.
Microscopic Features
- Pathognomonic cytologic change in all infarcts is coagulative (ischemic) necrosis of the affected area of tissue or organ. In cerebral infarcts, however, there is characteristic liquefactive necrosis.
- Some amount of hemorrhage is generally present in any infarct.
- At the periphery of an infarct, an inflammatory reaction is noted. Initially, neutrophils predominate but subsequently, macrophages and fibroblasts appear.
- Eventually, the necrotic area is replaced by fibrous scar tissue, which at times may show dystrophic calcification.
- In cerebral infarcts, the liquefactive necrosis is followed by gliosis, i.e. replacement by microglial cells distended by fatt material (gitter cells).
Question 14. Write a brief note on pulmonary embolism.
Answer:
Pulmonary embolism is the most common and fatal form of venous thromboembolism in which there is occlusion of the pulmonary arterial tree by thromboembolic.
Pulmonary embolism Etiology
Pulmonary emboli are most common in hospitalized or bedridden patients. The causes are:
- Thrombi originates from large veins of the lower legs, femoral and iliac.
- Less common sources include thrombi in varicosities of superficial veins of legs and pelvic veins.
Pulmonary embolism Pathogenesis
- If the thrombus is large, it is impacted at the bifurcation of the main pulmonary artery or may be found in the right ventricle or its outflow tract.
- More commonly there are multiple emboli or a large. embolus may be fragmented into many smaller emboli which then impacted a number of small vessels, particularly the lower lobes of the lungs.
- Rarely paradoxical embolism may occur by the passage of an embolus from the right heart into the left heart through an atrial or ventricular septal defect. In this way, pulmonary emboli may reach the systemic circulation.
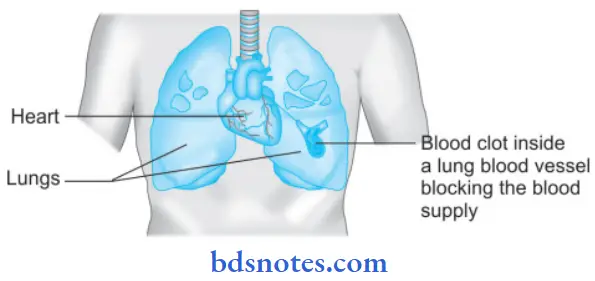
Consequences of Pulmonary Embolism
- Sudden death: Massive pulmonary embolism results in instantaneous death, without occurrence of chest pain or dyspnea. However, if the death is somewhat delayed, the clinical features resemble myocardial infarction, i.e. severe chest pain, dyspnea and shock.
- Acute cor pulmonale: Numerous small emboli may obstruct most of the pulmonary circulation resulting in acute right heart failure. Another mechanism is by the release of vasoconstrictor substances from platelets or by reflex vasoconstriction of pulmonary vessels.
- Pulmonary infarction: Obstruction of relatively small-sized pulmonary arterial branches may result in pulmonary infarction. The clinical features include chest pain due to fibrinous pleuritis, hemoptysis and dyspnea due to reduced functioning pulmonary parenchyma.
- Pulmonary hemorrhage: Obstruction of terminal branches (end arteries) leads to central pulmonary hemorrhage. The clinical features are hemoptysis, dyspnea, and less commonly, chest pain due to the central location of pulmonary hemorrhage. Sometimes, there may be concomitant pulmonary infarction.
- Resolution: Vast majority of small pulmonary emboli (60-80%) are resolved by fibrinolytic activity. These patients are clinically silent owing to bronchial circulation so that lung parenchyma is adequately perfused.
- Pulmonary hypertension, chronic cor pulmonale, and pulmonary arteriosclerosis: These are the sequelae of multiple small thromboembolic undergoing organization
rather than resolution.
Question 15. Write the difference between red and white infarct.
Answer:
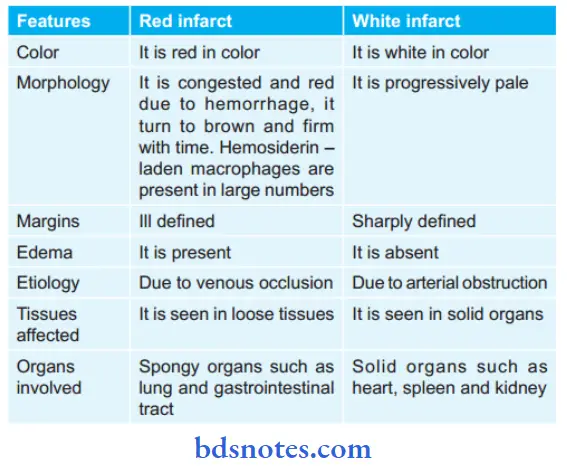
Question 16. Write a short note on edema.
Answer:
Edema is defined as abnormal and excessive accumulation of free fluid in the interstitial tissue spaces and serous cavities.
Edema Classification
1. Based on distribution and extent of involvement
- Localized: It is limited to an organ or limb, For Example. lymphatic edema, inflammatory edema, allergic edema, and pulmonary edema.
- Generalized: It is noticeable in the subcutaneous tissue, For Example. renal edema, cardiac edema and nutritional edema.
2. Based on fluid composition
- Transudate: It is more often the case, such as in edema of cardiac and renal disease.
- Exudate: Such as in inflammatory edema.
Pathogenesis of Edema
1. Decreased plasma oncotic pressure: It is exerted by the total amount of plasma proteins that tend to draw fluid into the vessels normally. A fall in the total plasma protein level (hypoproteinemia of less than 5 g/dl, mainly hypoalbuminemia), results in a lowering of plasma oncotic pressure in a way that it can no longer counteract the effect of the hydrostatic pressure of blood. This results in the increased outward movement of fluid from the capillary wall and decreased inward movement of fluid from the interstitial space causing edema. Hypoproteinemia usually produces generalized edema. Out of the various plasma proteins, albumin has four times higher plasma oncotic pressure than globulin; thus it is mainly hypoalbuminemia (albumin below 2.5 g/dl) that generally results in edema.
2. Increased capillary hydrostatic pressure: The hydrostatic pressure of the capillary is the force that normally tends to drive fluid through the capillary wall into the interstitial space by counteracting the force of plasma oncotic pressure. A rise in the hydrostatic pressure at the venular end of the capillary which is normally low to a level more than the plasma oncotic pressure results in minimal or no reabsorption of fluid at the venular end, consequently leading to edema.
3. Lymphatic obstruction: Normally, the interstitial flid in the tissue spaces escapes by way of lymphatics. Obstruction to the outflow of these channels causes localized edema, known as lymphedema.
4. Tissue factors: The two forces acting in the interstitial space—oncotic pressure of the interstitial space and tissue tension, are normally quite small and insignificant to counteract the effects of plasma oncotic pressure and capillary hydrostatic pressure respectively. However, in some situations, the tissue factors in combination with other mechanisms play a role in the causation of edema.
5. Increased capillary permeability: An intact capillary endothelium is a semipermeable membrane that permits the free flow of water and crystalloids but allows minimal passage of plasma proteins normally. However, when the capillary endothelium is injured by various ‘capillary poisons’ such as toxins and their products (For Example. histamine, anoxia, venoms, certain drugs, and chemicals), the capillary permeability to plasma proteins is enhanced due to the development of gaps between the endothelial cells causing leakage of plasma proteins into the interstitial fluid. This in turn causes reduced plasma oncotic pressure and elevated oncotic pressure of the interstitial fluid, consequently producing edema.
6. Sodium and water retention: The mechanism of edema by sodium and water retention in the extravascular compartment is best described in relation to derangement in the normal regulatory mechanism of sodium and water balance. Normally about 80% of sodium is reabsorbed by the proximal convoluted tubule under the influence of either intrinsic renal mechanism or extrarenal mechanism while retention of water is affected by the release of antidiuretic hormone. The possible factors responsible for causing edema by excessive retention of sodium and water in the extravascular compartment via stimulation of intrinsic renal and extrarenal mechanism as well as via release of ADH are:
- Reduced glomerular filtration rate in response to hypovolemia.
- Enhanced tubular reabsorption of sodium and consequently its decreased renal excretion.
- Increased filtration factor, i.e. increased filtration of plasma from the glomerulus.
- Decreased capillary hydrostatic pressure is associated with increased renal vascular resistance.
Consequences of Edema
Edema can compromise the cellular function in the following ways, i.e.
- Due to the expansion of interstitial space, there is an increase in diffusion distance for oxygen and other nutrients, which hampers cellular metabolism. An example is impaired gas exchange because of pulmonary edema.
- Expansion of interstitial space interferes with the removal of toxic byproducts of cellular metabolism.
Question 17. Write a short note on types of embolism.
Answer:
Embolism is the process of partial or complete obstruction of some part of the cardiovascular system by any mass carried in circulation. The mass is called as embolus.
Emboli may be of Various Types
- Depending upon the matter in the emboli:
- Solid, For Example. detached thrombi
- Liquid, For Example. fat globules
- Gaseous, For Example. air.
- Depending upon whether infected or not:
- Bland, when sterile
- Septic, when infected.
- Depending on the source of emboli:
- Cardiac emboli: From the left side of the heart. For Example. Emboli originating in the atrium and atrial appendages, infarct in the left ventricle, the vegetation of endocarditis.
- Arterial emboli: For Example. In systemic arteries in the brain, spleen, kidney, and intestine.
- Venous emboli: For Example. In pulmonary arteries.
- Lymphatic emboli: It can also occur sometimes.
- Depending upon the flow of blood:
- Paradoxical embolism: An embolus that is carried from the venous side of the circulation to the arterial side or vice versa.
- Retrograde emboli: An emboli which travel against the flow of blood is called a retrograde embolus.
Question 18. Write a short note on the pathogenesis of septic shock.
Or
Discuss the pathogenesis of septic shock.
Answer:
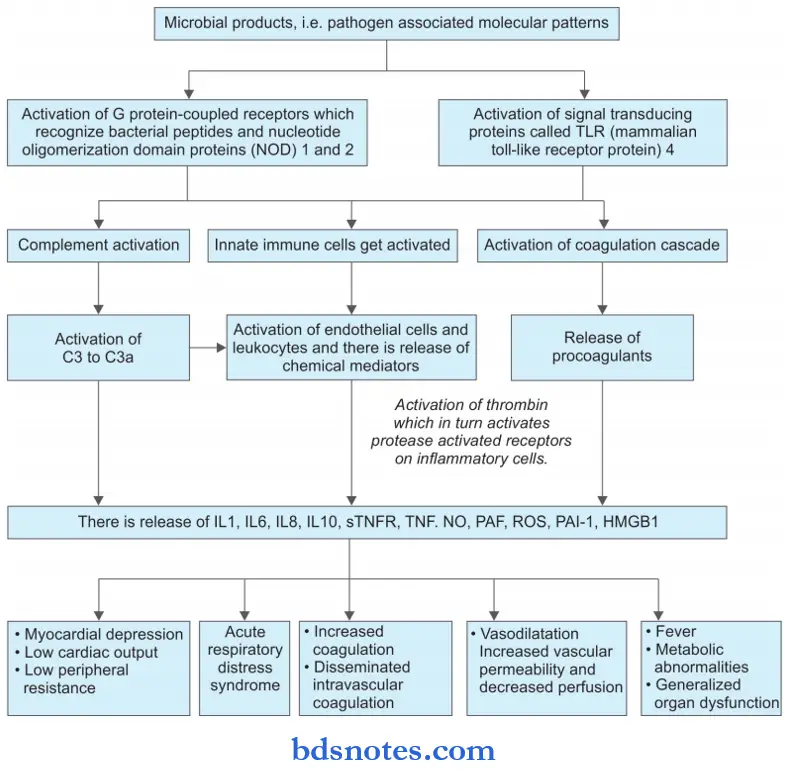
Question 19. Write a short note on factors involved in thrombus formation.
Answer:
Thrombosis is the process of formation of solid mass in circulation from the constituents of flowing blood, the mass itself is called as thrombus.
Factors Involved in Thrombus Formation
There are three major contributors:
- Endothelial injury: It can be secondary to:
- Myocarditis
- Myocardial infarction
- Cardiac surgery
- Ulcerated atherosclerotic plaque
- Infected valve disease
- Prosthetic valves
- Radiation injury
- Chemical agents such as smoking, hypercholesterolemia etc.
- Alteration in normal blood flow: It leads to
- Disruption of laminar flow
- Damage to endothelium
- Decreased hepatic clearance of activated coagulation factors
- Conditions predisposing to hypercoagulability.
It is divided into two, i.e. genetic and acquired factors. Some of the genetic factors which predispose to hypercoagulability are:
- Deficiency of anti-thrombotic factors, i.e. anti-thrombin 3, protein C and S, and defects in fibrinolysis.
- Increase in prothrombotic factors such as in factor 5 mutation, high level of factors 7, 9, 11, 8, Von Will brand factor, and fibrinogen.
Some of the acquired factors which predispose to hypercoagulability are:
- Venous stasis: It is due to prolonged immobilization and congestive cardiac failure.
- Increased platelet activation: In cancers, acute leukemias, myeloproliferative disorders, paroxysmal nocturnal hemoglobinuria, prosthetic cardiac valves, atrial fibrillation, etc.
- Due to increased hepatic synthesis of coagulation factors or reduced anticoagulant synthesis due to oral contraceptives and in pregnancy.
- Due to tissue injuries such as in surgery, fracture, and extensive burns.
Question 20. Write a short note on shock.
Or
Defie shock. What are the types of shock?
Answer:
Shock is defied as a clinical state of cardiovascular col-collapse characterized by an acute reduction of effective circulatory blood volume and inadequate perfusion of cells and tissues.
Classification and Etiology of Shock
1. Hypovolemic Shock
- Acute hemorrhage
- Dehydration from vomiting, diarrhea
- Burns
- Excessive use of diuretics
- Acute pancreatitis
2. Cardiogenic Shock
- Deficient emptying, For Example.
- Myocardial infarction
- Cardiomyopathies
- Rupture of the heart, ventricle, or papillary muscle
- Cardiac arrhythmias
- Deficient filing, For Example.
- Cardiac tamponade from hemopericardium
- Obstruction to the outflow, For Example.
- Pulmonary embolism
- Ball valve thrombus
- Tension pneumothorax
- Dissecting aortic aneurysm
3. Septic Shock
- Gram-negative septicemia (endotoxic shock), For Example. infection with E. coli, Proteus, Klebsiella, Pseudomonas, and Bacteroides
- Gram-positive septicemia (exotoxic shock), For Example. infection with streptococci, and pneumococci.
4. Other types
- Traumatic shock
- Severe injuries
- Surgery with marked blood loss
- Obstetrical trauma
- Neurogenic shock
- High cervical spinal cord injury
- Accidental high spinal anesthesia
- Severe head injury
- Hypoadrenal shock
- Administration of high doses of glucocorticoids
- Secondary adrenal insufficiency (For Example. in tuberculosis, metastatic disease, bilateral adrenal hemorrhage, idiopathic adrenal atrophy)
Shock Pathogenesis

Clinical Features of Shock
- Hypotension
- Cold clammy skin
- Rapid, thready pulse
- Shallow and sighing respiration
- Pale face, sunken eyes and weakness
- Uncontrolled sepsis-warm skin due to vasodilatation
- Urinary output less than 30 ml/hour
Stages of Shock
- An initial nonprogressive phase during which reflex compensatory mechanisms are activated and perfusion of vital organs is maintained
- A progressive stage characterized by tissue hypoperfusion and on the set of worsening circulatory and metabolic imbalances including acidosis
- An irreversible stage that sets in after the body has incurred cellular and tissue injury so severe that even if the hemodynamic defects are corrected, survival is not possible.
Morphologic Changes of Shock
The morphogenic changes in shock are due to hypoxia resulting in degeneration and necrosis.
- Hypoxic encephalopathy: Cerebral ischemia in compensated shock may produce an altered state of consciousness. In prolonged shock and cardiac arrest, the brain suffers from severe ischemic damage with loss of cortical function, comma, and a vegetative state. Dead and dying nerve cells are replaced by gliosis.
- Heart in shock: There are two important morphogenic changes in the heart in all types of shock
- Hemorrhage and necrosis: There may be small and large ischemic areas or infarcts particularly located in the subpericardial and subendocardial regions.
- Zonal lesion: There are opaque transverse contraction bands in myocytes near the intercalated disc.
- Shock lung: Due to dual blood supply lung are not affected by hypovolemic shock but is affected by septic shock.
- Lungs become heavy and wet.
- Changes of adult respiratory distress syndrome are seen.
- The changes include congestion, interstitial and alveolar edema, lymphocyte infiltration, and fibrin and platelet thrombi in the microvasculature.
- Shock kidney:
- Irreversible renal injury.
- The end result is generally anuria and death.
- Tubular lesions are seen referred to as acute tubular necrosis.
- Adrenal shock:
- Adrenal shows stress syndrome.
- This includes the release of aldosterone, glucocorticoid, and catecholamines like adrenaline. In severe shock, adrenal hemorrhage can occur.
- Liver in shock:
- Due to hypoxia vasodepressor material is released which causes vasodilatation.
- Focal necrosis, fatty liver, and impaired liver functions.
- Hemorrhagic gastroentropathy: Hyper fusion of alimentary tract.
Shock Complications
Life-threatening complications in shock are due to hypoxic cell injury which results in immune-inflammatory responses and activation of various cascades. Various complications of shockare:
- Acute respiratory distress syndrome
- Disseminated intravascular coagulation
- Acute renal failure
- Multiple organ dysfunction syndrome
With the progression of the condition, the patient can develop a stupor, coma, and finally death.
Question 21. Write a note on embolism.
Or
Write a short answer on embolism.
Answer:
Embolism is the process of partial or complete obstruction of some part of the cardiovascular system by any mass carried in the circulation; the transported intravascular mass detached from its site of origin is called an embolism.
Emboli may be of Various Types
- Depending upon the matter in the emboli, they can be:
- Solid: Detached thrombi (thromboembolic), atheromatous material, tumor cell clumps, tissue fragments, parasites, bacterial clumps, and foreign bodies.
- Liquid: Fat globules, amniotic fluid, bone marrow.
- Gaseous: Air, and other gases.
- Depending upon whether infected or not, they are called:
- Bland: when sterile.
- Septic: when infected.
- Depending upon the source of the emboli, they are classified as:
- Cardiac emboli from the left side of the heart, For Example. emboli originating from the atrium and atrial appendages, infarction in the left ventricle, and vegetations of endocarditis.
- Arterial emboli, For Example. in systemic arteries in the brain, spleen, kidney intestine.
- Venous emboli, For Example. in pulmonary arteries.
- Lymphatic emboli can also occur.
- Depending upon the flow of blood, two special types of emboli are mentioned:
- Paradoxical embolus: An embolus that is carried from the venous side of circulation to the arterial side or vice versa is called a paradoxical or crossed embolus, For Example. through arteriovenous communication such as in
patent foramen ovale, septal defect of the heart, and arteriovenous shunts in the lungs. - Retrograde embolus: An embolus that travels against the flow of blood is called a retrograde embolus, For Example. metastatic deposits in the spine from carcinoma prostate. The spread occurs by retrograde embolism through intraspinal veins which carry tumor emboli from large thoracic and abdominal veins due to increased pressure in body cavities, For Example. during
coughing or straining.
- Paradoxical embolus: An embolus that is carried from the venous side of circulation to the arterial side or vice versa is called a paradoxical or crossed embolus, For Example. through arteriovenous communication such as in
Question 22. Write a short note on pulmonary edema.
Answer:
Acute pulmonary edema is the most important form of local edema as it causes serious functional impairment but has special features.
- It differs from edema elsewhere in that the fluid accumulation is not only in tissue space but also in the pulmonary alveoli.
Pulmonary Edema Etiopathogenesis
Pulmonary edema can result from either the elevation of pulmonary hydrostatic pressure or the increased vascular permeability:
- Elevation in pulmonary hydrostatic pressure: In heart failure, there is an increase in the pressure in pulmonary veins which is transmitted to pulmonary capillaries. This leads to a disturbance in the balance between pulmonary hydrostatic pressure and the plasma oncotic pressure so that excessive fluid moves out of pulmonary capillaries into the interstitium of the lungs. Simultaneously endothelium of the pulmonary capillaries develops fenestrations permitting passage of plasma proteins and fluid into the interstitium. The interstitial fluid collected is cleared by the lymphatics. As the capacity of the lymphatics to drain the fluid is exceeded, the excess fluid starts accumulating in the interstitium, i.e. in the loose tissues around bronchioles, arteries, and lobular septa. Next follows the thickening of the alveolar walls because of the interstitial edema. Up to this stage, no significant impairment of gaseous exchange occurs.
- However, with a prolonged elevation of hydrostatic pressure and due to the high pressure of interstitial edema, the alveolar lining cells break and the alveolar air spaces are flooded with fluid driving the air out of the alveolus, thus seriously hampering the lung function.
- Examples of pulmonary edema by this mechanism are seen in left heart failure, mitral stenosis, pulmonary vein obstruction, thyrotoxicosis, cardiac surgery nephritic syndrome, and obstruction to the lymphatic outflow by tumor or inflammation.
- Increased vascular permeability: Vascular endothelium and alveolar epithelial cells get damaged leading to increased vascular permeability. Due to this, there is excessive fluid and plasma proteins leak out in interstitium and
in alveoli.
Pulmonary edema Pathology
Grossly
The lungs are heavy, moist, and sub crepitant. The cut surface exudes a frothy fluid.
Microscopically
- Alveolar spaces are congested.
- Initially excess fluid collects in interstitial lung spaces in septal walls. Later on, fluid fills the alveolar spaces.
- Edema fluid both in the interstitium and in alveolar spaces appears in eosinophilic, granular, and pink proteinaceous material which is admixed with some RBCs and
macrophages known as heart failure cells. - Alveolar edema is seen as brightly eosinophilic pink lines along the alveolar margin known as a hyaline membrane.
Question 23. Write a brief on nutmeg liver.
Answer:
Nutmeg liver is seen in chronic venous congestion (CVC) of the liver.
- It is called nutmeg because when CVC liver is examined, grossly the cut surface of the liver shows a red and yellow mottled appearance corresponding to a congested center of lobules and fatt peripheral zone respectively.
- It is one of the very characteristic features of diagnosing or getting the clue for CVC liver.
Question 24. Write a short note on thromboembolism.
Answer:
An embolus is a detached intravascular solid, liquid, or gaseous mass that is carried by the blood to a site distant from its point of origin. When emboli represent some part of a dislodged thrombus it is known as thrombo-embolism.
Thromboembolism is of two types, i.e. systemic or arterial and pulmonary.
Systemic or Arterial Thromboembolism
- Systemic thromboembolism refers to emboli, traveling within the arterial circulation.
- Most of them arise from intracardiac mural thrombi, two-thirds of which are associated with left ventricular wall infarcts and another quarter with dilated left atria.
- The remainder largely originates from aortic aneurysms, thrombi on ulcerated atherosclerotic plaques, or fragmentation of valvular vegetation, while only a small fraction is due to paradoxical emboli, venous emboli, tend to lodge primarily in one vascular bed (the lung), arterial emboli can travel to a wide variety of sites; the site of an arrest depends on the point of origin of the thromboembolic and the volume of blood flow through the downstream tissues.
- The major sites for arteriolar embolization are the lower extremities and the brain, with the intestines, kidneys, spleen, and upper extremities involved to a lesser extent.
- The consequences of systemic emboli depend on any collateral vascular supply in the affected tissue, the tissue’s vulnerability to ischemia, and the caliber of the vessel occluded; in general, however, arterial emboli cause infarction of tissues in the distribution of the obstructed vessel.
Venous Thromboembolism
- Venous emboli may arise from the following sources, i.e.
- Deep vein thrombosis of lower legs
- Thrombi in pelvic veins
- Thrombi in veins of upper limbs
- Thrombosis in the cavernous sinus of the brain
- Thrombi in the right side of the heart
The most significant effect of venous embolism is obstruction of pulmonary arterial circulation leading to pulmonary embolism.
Question 25. Describe septicemia shock.
Answer:
Septicemia Shock
- It is also known as septic shock.
- Septic shock is a vasodilator shock wherein there is peripheral vasodilation causing hypotension which is resistant to vasopressors.
Septicemia Shock Types
Septic shock is of two types i.e.
- Gram-positive septic shock: It is caused due to exotoxin produced by Gram-positive bacteria like Cl. tetani, staphylococci, and streptococci. There is the presence of fluid loss and hypotension.
- Gram-negative septic shock: Gram-negative bacteria cause endotoxemia. Urinary, gastrointestinal, bacillary, and respiratory foci are common.
Pathogenesis of Septic Shock
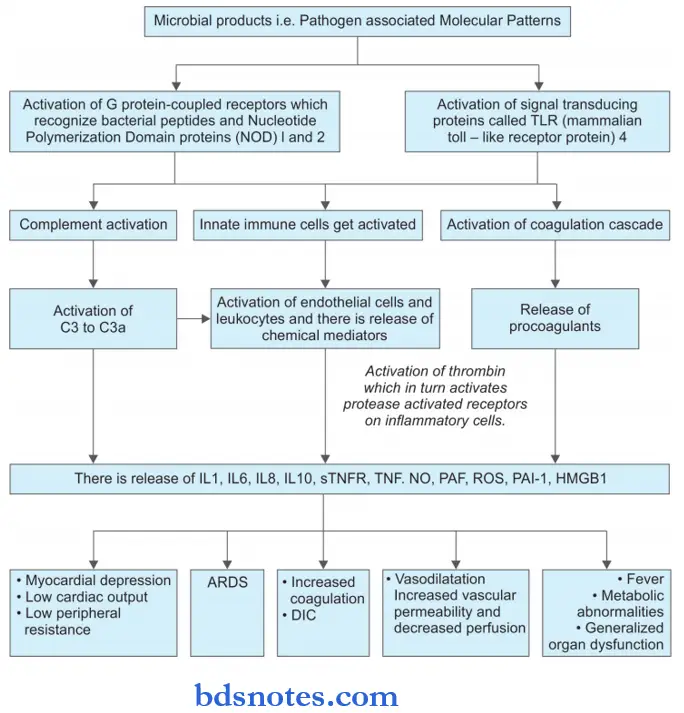
Septicemia Shock Clinical Features
Based on stages of septic shock:
- Hyperdynamic or warm shock
- It is a reversible stage.
- The patient has fever, tachycardia, and tachypnea
- The pyogenic response is intact
- Hypodynamic or cold septic shock
- The pyogenic response is lost
- The patient is in decompensated shock.
- Presence of anuria, cyanosis, jaundice, cardiac depression, pulmonary edema, hypoxia, drowsiness, coma and death.
Question 26. Write a brief on embolism.
Answer:
Fat Embolism
Obstruction of arterioles and capillaries by fat globules constitutes fat embolism. If the obstruction in the circulation is by fragments of adipose tissue, it is called a fat-tissue embolism.
Embolism Etiology
Causes of fat embolism may be traumatic and non-traumatic:
Traumatic Causes
- Trauma to bones is the most common cause of fat embolism For Example. in fractures of long bones leading to passage of fatty marrow in circulation, concussions of bones, after orthopedic surgical procedures, etc.
- Trauma to soft tissue, e.g. laceration of adipose tissue and in puerperium due to injury to pelvic fatty tissue.
Non-traumatic Causes
- Extensive burns
- Diabetes mellitus
- Fatt liver
- Pancreatitis
- Sickle cell anemia
- Decompression sickness
- Inflammation of bones and soft tissues
- Extrinsic fat or oils introduced into the body
- Hyperlipidemia
- Cardiopulmonary bypass surgery.
Embolism Pathogenesis
Pathogenesis of fat embolism is explained by the following mechanisms which may be acting singly or in combination:
- Mechanical theory: Mobilization of fluid fat may occur following trauma to the bone or soft tissues. Fat globules released from the injured area may enter venous circulation and finally, most of the fat is arrested in the small vessels in the lungs. Some of the fat globules may further pass through the lungs and enter into the systemic circulation to lodge in other organs.
- Emulsion instability theory: This theory explains the pathogenesis of fat embolism in non-traumatic cases. According to this theory, fat emboli are formed by aggregation of plasma lipids (chylomicrons and fatty acids) due to disturbance in the natural emulsification of fat.
- Intravascular coagulation theory: In stress, the release of some factors activates disseminated intravascular coagulation (DIC) and aggregation of fat emboli.
- Toxic injury theory: According to this theory, the small blood vessels of the lungs are chemically injured by high plasma levels of free fatty acid, resulting in increased vascular permeability and consequent pulmonary edema.
Gas Embolism
Air, nitrogen, and other gases can produce bubbles within the circulation and obstruct the blood vessels causing damage to tissue. Two main forms of gas embolism are air embolism and decompression sickness.
Air Embolism
Air embolism occurs when air is introduced into venous or arterial circulation.
Venous Air Embolism
Air may be sucked into systemic veins under the following circumstances:
- Operations on the head and neck, and trauma
- Obstetrical Operations and trauma during childbirth
- Intravenous infusion of blood and fluid
- Angiography
Arterial Air Embolism
Entry of air into a pulmonary vein or its tributaries may occur in the following conditions:
- Cardiothoracic surgery and trauma
- Paradoxical air embolism
- Arteriography
Decompression Sickness
This is a specialized form of gas embolism known by various names such as caissons disease, divers’ palsy, or aeroembolism.
Pathogenesis
Decompression sickness is produced when the individual decompresses suddenly, either from high atmospheric pressure to a normal level or from normal pressure to low atmospheric pressure. In divers, workers in caissons (diving-bells), of shore drilling and tunnels, who descend to high atmospheric pressure, increased amount of atmospheric gases are dissolved in the blood, and tissue fluids. When such an individual ascends too rapidly i.e. comes to normal level suddenly from high atmospheric pressure, the gases come out of the solution as minute bubbles, particularly in fatty tissues which have an affinity for nitrogen. These bubbles may coalesce together to form large emboli.
In aeroembolism, seen in those who ascend to high altitudes or air flight in unpressurized cabins, the individuals are exposed to sudden decompression from low atmospheric pressure to normal levels. This results in similar effects as in divers and workers in caissons.
Clinical Effects
The clinical effects are acute or chronic.
Acute Form
It occurs due to acute obstruction of small blood vessels in the vicinity of joints and skeletal muscles. The condition is clinically characterized by the following:
- The bends’, as the patient doubles up in bed due to acute pain in joints, ligaments, and tendons.
- The chokes occur due to the accumulation of bubbles in the lungs, resulting in acute respiratory distress.
- Cerebral effects may manifest in the form of vertigo, coma, and sometimes death.
Chronic Form
The chronic form is due to foci of ischemic necrosis throughout the body, especially the skeletal system. Ischemic necrosis may be due to embolism per se, but other factors such as platelet activation, intravascular coagulation, and hypoxia might contribute.
The features of the chronic form are as under:
- Avascalar necrosis of bones, For Example. head of the femur, tibia, and humerus.
- Neurological symptoms may occur due to ischemic necrosis in the central nervous system. These include paresthesia and paraplegia.
- Lung involvement in the form of hemorrhage, edema, emphysema, and atelectasis may be seen. These result in dyspnea, nonproductive cough, and chest pain.
- Skin manifestations include itching, patchy erythema, cyanosis, and edema.
- Other organs like parenchymal cells of the liver and pancreas may show lipid vacuoles.
Amniotic Fluid Embolism
- This is the most serious, unpredictable, and unpreventable cause of maternal mortality. During labor and in the immediate postpartum period, the contents of amniotic fluid may enter the uterine veins and reach the right side of the heart resulting in fatal complications.
- Its onset is characterized by sudden severe dyspnea, cyanosis, and hypotensive shock which is followed by seizures and coma.
- The mechanism by which these amniotic fluid contents enter the maternal circulation is not clear. Possibly, they gain entry either through tears in the myometrium and endocervix, or the amniotic flid is forced into uterine
sinusoids by vigorous uterine contractions. - It has a mortality rate of 20 to 40% of cases.
Embolism Atheroembolism
Atheromatous plaques, especially from the aorta, may get eroded to form atherosclerotic emboli which are then lodged in medium—sized and small arteries. These emboli consist of cholesterol crystals, hyaline debris, and calcified material, and may evoke foreign body reactions at the site of lodgement.
Tumor Embolism
Malignant tumor cells invade the local blood vessels and may form tumor emboli to be lodged elsewhere, producing metastatic tumor deposits. Examples are clear cell carcinoma of kidney, carcinoma of the lung, malignant melanoma, etc.
Miscellaneous Emboli
Various other endogenous and exogenous substances may act as emboli. These may include the following:
- Fragments of tissue
- Placental fragments
- Red cell aggregates (sludging)
- Bacteria
- Parasites
- Barium emboli following enema
- Foreignbodies, For Example. needles, talc, sutures, bullets, catheters, etc
Question 27. Describe important types of edema. Write in detail.
Answer:
Important Types of Edema
The following are the important types of edema:
- Renal edema
- Cardiac edema
- Pulmonary edema
- Cerebral edema.
Renal Edema
Generalized edema occurs in various diseases of renal origin, i.e. nephritic syndrome, glomerulonephritis, and in acute tubular injury.
Edema Pathogenesis
Edema in Nephrotic Syndrome
As there is persistent and heavy proteinuria occurs in nephrotic syndrome there is hypoalbuminemia which leads to decreased plasma oncotic pressure which causes severe generalized edema. Hypoalbuminemia leads to a fall in plasma volume which activates the rennin angiotensin–aldosterone mechanism which causes retention of sodium and water which persists till albuminuria continues.
Edema in Nephritic Syndrome
This occurs in glomerulonephritis. Nephritic edema occurs due to excessive reabsorption of sodium and water in renal tubules through the renin-angiotensin-aldosterone system.
Edema in Acute Tubular Injury
This occurs following the shock or toxic chemicals which results in gross edema of the body. Damaged tubules lost their capacity for selective reabsorption and concentration of glomerular filtrate which causes increased reabsorption of water and oliguria and edema to occur.
Cardiac Edema
Generalized edema is seen in right-sided heart failure and in congestive cardiac failure.
Cardiac Pathogenesis
- Hypovolemia occurs due to reduced cardiac output which leads to stimulation of intrinsic renal and extra-renal hormonal mechanisms along with ADH secretion which causes sodium and water retention with edema.
- Heart failure causes an increase in central venous pressure which gets transmitted to the venous end of capillaries increasing capillary hydrostatic pressure and consequent transudation called as back pressure hypothesis.
- Chronic hypoxia injures the capillary wall leading to increased capillary permeability which causes edema and is known as the forward pressure hypothesis. This is not accepted.
- Cardiac edema depends solely on the edema. In an ambulatory patient, edema occurs in the lower extremities while in bedridden patients it occurs in the sacral and genital areas.
Pulmonary Edema
It occurs from left heart failure. Fluid accumulation occurs in tissue spaces and pulmonary emboli.
Pulmonary Pathogenesis
Pulmonary edema occurs either from elevation of pulmonary hydrostatic pressure or increased capillary permeability.
1. Elevation in Pulmonary Hydrostatic Pressure
In heart failure, there is an increase in the pressure of pulmonary veins which gets transmitted to pulmonary capillaries.
This causes an imbalance between the pulmonary hydrostatic pressure and plasma oncotic pressure so the excessive fluid moves out of pulmonary capillaries inside the interstitium of the lungs.
Collected interstitial fluid gets cleared by lymphatics present around the bronchioles. Since the draining capacity of lymphatics for draining the fluid gets exceeded the excess fluid accumulates in the interstitium.
Elevation of hydrostatic pressure due to high pressure of interstitial edema the alveolar lining cells to break and alveolar air spaces to get flooded with the flid. It is commonly seen in left heart failure, mitral stenosis, pulmonary vein obstruction, thyrotoxicosis, etc.
2. Increased Vascular Permeability
Vascular endothelium and alveolar epithelial cells become damaged leading to increased vascular permeability which causes excessive fluid and plasma proteins leakage inside the interstitium and then in the alveoli.
This is seen in fulminant pulmonary and extrapulmonary infections, shock, radiation injury, etc.
3. Acute High Altitude Edema
People climbing to a high altitude suddenly without halt and without waiting for acclimatization to set in suffer from serious circulatory and respiratory ill effects.
These changes lead to the appearance of edema in the lungs, congestion, and widespread minute hemorrhages. Here anoxia damages the pulmonary vessels.
Cerebral Edema
Inside the brain, the blood-brain barrier causes fluid electrolyte exchange.
Cerebral edema is of three types viz:
- Vasogenic edema
- Cytotoxic edema
- Interstitial edema.
Vasogenic Edema
It results due to increased filtration pressure or increased capillary permeability. This edema is prominent at cerebral contusions, infarcts, brain abscesses, and in a few tumors.
Cytotoxic Edema
In this blood-brain barrier is intact and fluid gets accumulated intracellularly. It occurs due to disturbance in cellular osmoregulation as seen in some metabolic derangements, and acute hypoxia with some toxic chemicals.
Interstitial Edema
It occurs when an excessive fluid crosses the ependymal lining of the ventricles and accumulates in periventricular white matter. It is seen in non-communicating hydrocephalus.
Question 28. Write notes on gross and microscopic appearances of infarction of the kidney.
Answer:
Gross Appearance of Kidney Infarct
Following is the gross appearance of kidney infarcts:
- Renal infarcts are multiple and can be bilateral.
- These are pale in appearance and are wedge-shaped with the base lying under the capsule and the apex pointing towards the medulla.
- A narrow rim of preserved renal tissue is seen under the capsule which is spared due to blood supply from capsular vessels.
- The cut surface of renal infarct during the first 2 to 3 days appears red.
- On the fourth day center become pale yellow in color.
- After one week infarct is anemic and gets depressed below the surface of the kidney.
Microscopic Appearance of Kidney Infarct
- There is the presence of coagulative necrosis of renal parenchyma, i.e. ghosts of renal tubules and glomeruli without intact nuclei and cytoplasmic content are seen.
- The margin of kidney infarct shows acute inflammatory reaction but later on macrophages and furious tissue predominate.
Question 29. Write a short note on infarction.
Answer:
Infarction is the process of tissue necrosis resulting from some form of circulatory insufficiency.
Infarction Etiology
- Infarcts are caused by an interruption in arterial blood supply known as ischemic necrosis.
- Venous obstruction can lead to infarcts.
- Sudden, complete and continuous occlusion leads to infarcts
- Infarction can be caused by non-occlusive circulatory insufficiency.
Infarction Pathogenesis
- As there is an obstruction of blood supply localized hyperemia occurs due to local anoxemia.
- In a few hours, the affected part becomes swollen because of edema and hemorrhage. Hemorrhage is more marked in the lungs and spleen and less marked in the kidneys and heart.
- Cloudy swelling and degeneration occur early and death of cells occurs in 24 to 48 hours.
- There is progressive proteolysis of necrotic tissue and lysis of RBCs.
- An acute inflammatory reaction and hyperemia are seen in surrounding tissues in response to proteolysis.
- Blood pigments and hemosiderin liberated from by lysis of RBCs deposit in the infarct. During this stage, infarcts become pale grey because of the loss of RBCs.
- Now there is progressive ingrowth of granulation tissue from the margin of the infarct so that the infarct is replaced by a fibrous scar. Dystrophic calcification occurs at times.
Infarction Histopathology
- A characteristic feature is coagulative necrosis of the affected area. It consists of some hemorrhage too.
- At the periphery there is the presence of an inflammatory reaction consisting of neutrophils initially and macrophages as well as fibroblasts appear later on.
- Most of the infarcts are replaced by fibrous scar tissue.
Question 30. Write a short note on the pathogenesis of edema.
Answer:
The following six mechanisms may be operating singly or in combination to produce edema:
Pathogenesis of Edema
1. Decreased plasma oncotic pressure: Plasma oncotic pressure is exerted by the total amount of plasma proteins that tend to draw fluid into the vessels normally.
A fall in the total plasma protein level (hypoproteinemia of less than 5 g/dl, mainly hypoalbuminemia), results in a lowering of plasma oncotic pressure in a way that it can no longer counteract the effect of hydrostatic pressure of blood.
This results in increased outward movement of fluid from the capillary wall and decreased inward movement of fluid from the interstitial space causing edema. Hypoproteinemia usually produces generalized edema.
Out of the various plasma proteins, albumin has four times higher plasma oncotic pressure than globulin; thus it is mainly hypoalbuminemia (albumin below 2.5 g/dl) that generally results in edema.
2. Increased capillary hydrostatic pressure: The hydrostatic pressure of the capillary is the force that normally tends to drive fluid through the capillary wall into the interstitial space by counteracting the force of plasma oncotic pressure.
A rise in the hydrostatic pressure at the venular end of the capillary which is normally low to a level more than the plasma oncotic pressure results in minimal or no reabsorption of fluid at the venular end, consequently leading to edema.
3. Lymphatic obstruction: Normally, the interstitial fulid in the tissue spaces escapes by way of lymphatics. Obstruction to the outflow of these channels causes localized edema, known as lymphedema.
4. Tissue factors: The two forces acting in the interstitial space, i.e. oncotic pressure of the interstitial space and tissue tension, are normally quite small and insignificant to counteract the effects of plasma oncotic pressure and capillary hydrostatic pressure respectively.
However, in some situations, the tissue factors in combination with other mechanisms play a role in the causation of edema.
5. Increased capillary permeability: An intact capillary endothelium is a semipermeable membrane that permits the free flow of water and crystalloids but allows minimal passage of plasma proteins normally.
However, when the capillary endothelium is injured by various ‘capillary poisons’ such as toxins and their products (For Example. histamine, anoxia, venoms, certain drugs, and chemicals).
The capillary permeability to plasma proteins is enhanced due to the development of gaps between the endothelial cells causing leakage of plasma proteins into the interstitial fluid.
This, in turn, causes reduced plasma oncotic pressure and elevated oncotic pressure of the interstitial fluid, consequently producing edema.
6. Sodium and water retention: The mechanism of edema by sodium and water retention in the extravascular compartment is best described in relation to derangement in the normal regulatory mechanism of sodium and water balance.
Normally about 80% of sodium is reabsorbed by the proximal convoluted tubule under the influence of either intrinsic renal mechanism or extrarenal mechanism while retention of water is affected by the release of antidiuretic hormone.
The possible factors responsible for causing edema by excessive retention of sodium and water in the extravascular compartment via stimulation of intrinsic renal and extrarenal mechanism as well as via release of ADH are:
- Reduced glomerular filtration rate in response to hypovolemia.
- Enhanced tubular reabsorption of sodium and consequently decreased renal excretion.
- Increased filtration factor, i.e. increased filtration of plasma from the glomerulus.
- Decreased capillary hydrostatic pressure is associated with increased renal vascular resistance.
Question 31. Write the pathophysiology of thrombosis. Describe the clinical effects.
Answer:
Pathophysiology of Thrombosis
Pathophysiology of thrombosis describes thrombogenesis in relation to the normal hemostatic mechanism.
Human beings consists of a system in which blood remains in its fluid state during normal condition and this system also act as a guard to stop thrombosis and hemorrhage.
The primary influence over the formation of a thrombus is known as Virchow’s triad. It consists of endothelial injury, altered blood flow, and hypercoagulability of blood.
Endothelial Injury
- Blood vessel integrity is important for maintaining the normal flow of blood.
- Injurytobloodvesselleadstoexposureofthesubendothelial connective tissue which a thrombogenic and play an important role in initiating hemostasis and thrombosis.
- Endothelial injury which leads to thrombogenesis occurs under diabetes mellitus, myocardial infarction, arterial diseases, etc.
- Vascular injury causes loss of epithelium which exposes extracellular material, adhesion of the platelets, the release of tissue factors, and depletion of prostaglandins and plasminogen activators which leads to the formation of thrombus.
- Any disturbance in the balance of prothrombotic and antithrombotic mechanisms of the endothelium of the vessel influences the local clotting mechanism.
- Endothelial dysfunction can lead to the release of more amount of more procoagulant factors which are plasminogen activator inhibitors, various tissue factors, etc.
Altered Blood Flow
- Normal axial flow of blood in it central stream consists of leucocytes and red blood cells. Platelets exist in a slow-moving laminar stream while which is adjacent to the central stream and the peripheral stream consists of slow-moving cell-free plasma close to the endothelial layer.
- Both turbulence and stasis occur in normal axial blood flow and disturb it.
- When the speed of flow of blood slows down blood cells containing platelets marginate toward the periphery and form a pavement close to the endothelium.
- As stasis leads to higher release of oxygen from the blood, turbulence injures the endothelium which leads to the deposition of platelets and fibrin.
- Turbulence causes the formation of arterial and cardiac thrombi while stasis leads to the formation of venous thrombi.
Hypercoagulability of Blood
- Hypercoagulability is the alteration in the coagulation pathway that leads to thrombosis.
- It occurs due to the following changes in the composition of blood:
- Increase in coagulation factors such as fibrinogen, prothrombin, factors 7a, 8a, and 10a.
- Increase in platelet count and its adhesiveness.
- Decrease in levels of coagulation inhibitors, i.e. antithrombin 3, firin’ split products.
Clinical Effects of Thrombus
Clinical effects depend on the site of the thrombi, its rapidity of formation, and the nature of the thrombi.
- Cardiac thrombi: If large thrombi are present in the heart they can cause sudden death due to mechanical obstruction of blood flow or via thromboembolism to vital organs:
- Arterial thrombi: It can lead to ischemic necrosis of the affected part which can cause gangrene. Sudden death can occur if there is thrombosis of the coronary artery.
- Venous thrombi: It can produce the following effects:
- Thromboembolism
- Edema of the area which is drained
- Poor wound healing
- Skin ulcer
- Painful thrombosed vein or thrombophlebitis
- Painful white leg caused by the file of femoral venous thrombosis in postpartum patients
- Thrombophlebitis migrants in the cancer
Capillary thrombi: The presence of microthrombi in the microcirculation can lead to disseminated intravascular coagulation.

Leave a Reply