Gypsum Products
Products of gypsum are used extensively in dentistry. Gypsum was found in mines around the city of Paris, so it is also called plaster of Paris. This is a misnomer as gypsum is found in most countries.
The mineral gypsum CaSO4. 2H2O is usually white to yellowish-white in color and is found as a compact mass. Gypsum is also an industrial byproduct. For centuries gypsum has been used for construction purposes and making statues.
Alabaster, a form of gypsum that is white, was used for building in ancient times. Besides dentistry, gypsum is also used in orthopedics for splinting fractured bones.
Read And Learn More: Basic Dental Materials Notes
Gypsum Products Applications
- Impression plaster was used extensively in the past for impressions of the mouth and face.
- Various types of plasters are used to make molds, casts, and dies over which dental prostheses and restorations are made.
- To attach casts to an articulator.
- For bite registration (e.g., to record centric jaw relation).
- Dental investments Plaster mixed with silica is known as a dental investment. They are used to form refractory molds into which molten metal is cast.
Gypsum Products Supplied As
Powders of various colors in small preweighed sachets, in medium-sized bags or containers, or large bags, sacks, or bins (bulk).
Gypsum Products Classification
Type 1—Dental plaster for impressions
Type 2—Dental plaster
- Class 1 – for mounting
- Class 2 – for models
Type 3—Dental stone for models
Type 4—Dental stone (high strength, low expansion) for dies
Type 5—Dental stone (high strength, high expansion) for dies
Type 1 Or Dental plaster, Impression
Impression plaster was one of the earliest impression materials in dentistry. Because of its rigidity (not elastic), it often had to be fractured to remove it from undercut areas in the mouth. The fractured pieces were then reassembled outside and a cast was poured.
Since the introduction of better materials, it is rarely used as an impression material. Currently, it is more useful as a bite registration material. Impression plaster may be flavored to make it more acceptable to the patient. It is colored to help the dentist and technician distinguish between the cast material and the impression.
Impression plaster, sometimes, contains potato starch to make it soluble. After the cast has hardened, the impression and cast are put in hot water. The starch swells and the impression disintegrates, making it easy to separate the cast. This type is often called ‘soluble plaster’.
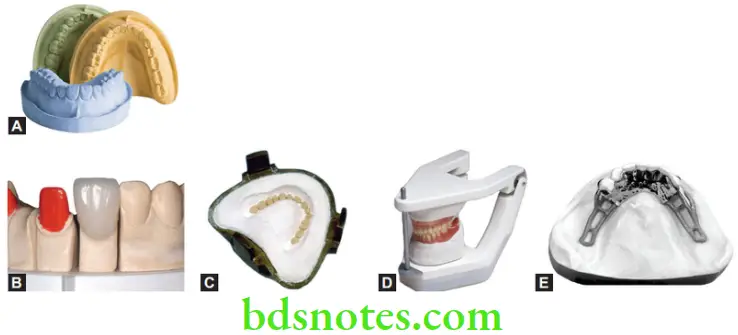
Uses
- For making impressions in complete dentures and maxillofacial prosthetics (not used currently for this purpose).
- Bite registration material.
Ideal Requirements
- The setting time should be under accurate control. The dentist must have sufficient time to mix, load the impression tray, carry the loaded tray to the patient’s mouth, and place it in position.
- However, once in position, the plaster should harden promptly so that there is minimum discomfort to the patient. The setting time desirable is 3 to 5 minutes.
- For better accuracy, the setting expansion should be low. Both setting time and expansion are controlled by modifiers (accelerators and retarders) added by the manufacturers.
- The plaster should have enough strength to fracture cleanly without crumbling to facilitate removal from undercuts.
Composition
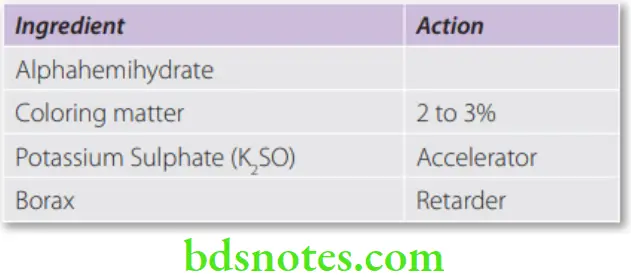
Dental plaster + K2SO4 + Borax + Coloring and flavoring agents.
Type 2 Or Dental Plaster, Model, Mounting
Synonyms Model plaster, laboratory plaster, mounting plaster.
The International Standards Organization (ISO 6873:2013) has classified Type 2 plaster into 2 subtypes—Class I (for mounting) and Class 2 (for models).
Uses
- For making study casts and models.
- To make molds for curing dentures.
- For mounting casts on the articulator.
Requirements of an ideal cast material
- It should set rapidly but give adequate time for manipulation.
- It should be set to a very hard and strong mass.
- It should flow into all parts of the impression and reproduce all the minute details.
- It should neither contract nor expand while setting.
- After setting, it should not warp or change shape.
- It should not lose its strength when subjected to molding and curing procedures.
Composition
Contains beta hemihydrate and modifiers.
Type 3 Dental Stone, model
Synonym Class I stone or Hydrocal.
Uses
For preparing master casts and making molds.
Composition
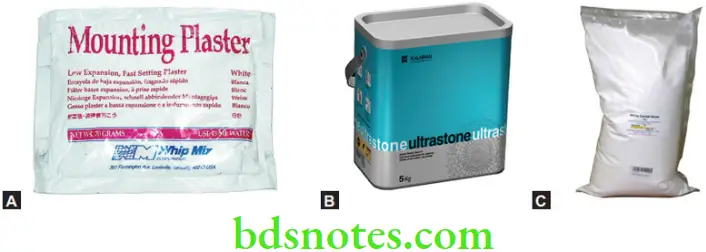
Some commercial dental stones contain a small amount of beta hemihydrate to provide a mix of smoother consistency.
A stone with a setting time established by the addition of proper quantities of both accelerator and retarder is called ‘balanced stone’. Typical accelerators are potassium sulfate and potassium sodium tartrate (Rochelle Salts). Typical retarders are sodium citrate and sodium tetraborate decahydrate (Borax).
- The compressive strength varies from 3000 to 5000 psi.
- The setting expansion of dental stones is 0.06% to 0.12%.
- Hardness: 82 RHN.
Type 4 Or Dental stone, die, high strength, low expansion
Synonyms Class 2 stone, die stone, densite, improved stone.
Uses
- Die stone is the strongest and hardest variety of gypsum products. It is used when high strength and surface hardness are required. Uses include model bases, CAD/CAM dies, and dies for fabricating inlay, crown, and bridge wax patterns.
- A thick mix is prepared as per the manufacturer’s instruction and vibrated into a rubber base impression. The base for such a model is poured in dental stone or dental plaster. Die stone should be left for twenty-four hours to gain maximum hardness and the cast should be separated one hour after pouring. The abrasion resistance of die stone is not as high as other die materials like epoxy resin.
- Recent revisions of the ISO (2013) have included additional requirements for Type 4 stone to reflect the introduction of new technologies like CAD/CAM.
Type 5 OR Dental Stone, Die, High Strength, High Expansion
It is the most recent gypsum product having a higher compressive strength than Type 4 stone. Improved strength is attained by making it possible to lower the w/p ratio even further.
Setting expansion has been increased from a maximum of 0.10 to 0.30%. This is to compensate for the shrinkage of base metal alloys, during solidification (see Casting Alloys).
Hard Rock, Jade Rock Resinrock XL5 (by Whipmix), and Denflo-HX are examples of Type 5 stone.
Uses
To prepare dies with increased expansion.

Manufacture of Gypsum Products
The process of heating gypsum for the manufacture of plaster is known as calcination. Mined gypsum is ground and heated. When heated, gypsum (calcium sulfate dihydrate) loses part of its water of crystallization and changes to calcium sulfate hemihydrate. On further heating, the remaining water of crystallization is lost. First, hexagonal anhydrite (soluble anhydrite) is formed. Later, orthorhombic anhydrite (insoluble anhydrite) is formed.
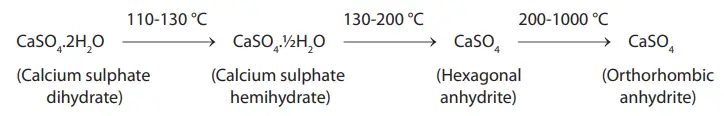
Alpha and beta hemihydrate
Depending on the method of calcination, there are two forms of hemihydrates.
- Beta hemihydrate (plaster)
- Alpha hemihydrate (stone)
- Alpha-modified hemihydrate (die stone)
Manufacture of dental plaster
Gypsum is ground and heated in an open kettle on a kiln at a temperature of 110 to 130 °C. The process is called dry-calcination. β type of crystals are formed.
Microscopically Fibrous aggregate of fine crystals with capillary pores. They are then ground to breakup the needlelike crystals. This improves packing.
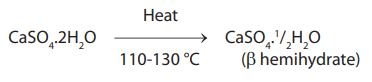
Manufacture of dental stone
Gypsum is calcined under steam pressure in an autoclave at 120 to 130 °C at 17 lbs/sq. inch for 5 to 7 hours. Thus, the product obtained is much stronger and harder than β hemihydrate.

Microscopically Cleavage fragments and crystals in the form of rods and prisms.
Manufacture of high-strength (α modified) stone
The gypsum is calcined by boiling it in a 30% calcium chloride solution. The chlorides are then washed away or autoclaved in the presence of sodium succinate 0.5%.
These particles are the densest of all three types. After controlled grinding, these powders have an even higher apparent density and yield a stronger set.
Microscopically cuboidal in shape.
Setting Reaction
When the plaster is mixed with water it takes up one and a half molecules of water, i.e., it regains its water of crystallization and becomes calcium sulfate dihydrate.
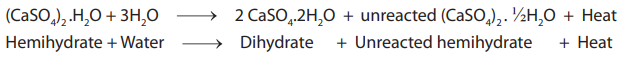
The reaction is exothermic and is the same for all gypsum products. The amount of water required to produce a workable mix varies between the products.
As evident from the above reaction not all of the hemihydrate converts to dihydrate. The amount of conversion is dependent on the type of stone.
The highest conversion rate is seen in plaster (90%). In Type 4 and 5 stone the dihydrate content is about 50%.
Theories Of Setting
Three theories have been proposed.
- Colloidal theory
- Hydration theory
- Dissolution – precipitation theory
Colloidal Theory
- The theory proposes that when mixed with water, plaster enters into a colloidal state through a sol-gel mechanism. In the sol state, hemihydrate combines with water (hydrates) to form dihydrate. As the water is consumed, the mass turns to a ‘solid gel’.
Hydration theory
- The hydration theory suggests that rehydrated plaster particles join together through hydrogen bonding to the sulfate groups to form the set material.
Dissolution–precipitation theory (Crystalline theory)
This theory is more widely accepted. According to the theory, the plaster dissolves and reacts to form gypsum crystals which interlock to form the set solid. The setting reaction is explained on the basis of the difference in solubility of hemihydrate and dihydrate. Hemihydrate is four times more soluble than dihydrate.
- When hemihydrate is mixed in water, it forms a fluid workable suspension.
- Hemihydrate dissolves until it forms a saturated solution.
- Some dihydrate is formed due to the reaction. The solubility of dihydrate is much less than hemihydrate, the saturated hemihydrate is supersaturated with respect to the dihydrate. All supersaturated solutions are unstable. So the dihydrate crystals precipitate out.
- As the dihydrate precipitates out, the solution is no longer saturated with hemihydrate and so it continues to dissolve. The process continues until no further dihydrate precipitates out of the solution.
Initially, there is a little reaction and thus little or no rise in temperature. This time is referred to as the induction period. As the reaction proceeds, gypsum is formed in the form of needle-like clusters, called spherulites. Continued growth and intermeshing of crystals of gypsum leads to thickening and hardening of the mass into a strong solid structure.
The Microstructure of Set Gypsum
The set material consists of an entangled aggregate of gypsum crystals having lengths of 5 to 10 µm. Two distinct types of microscopic porosity can be seen in the mass.
- Microporosity is caused by residual unreacted water. These voids are spherical and occur between clumps of gypsum crystals.
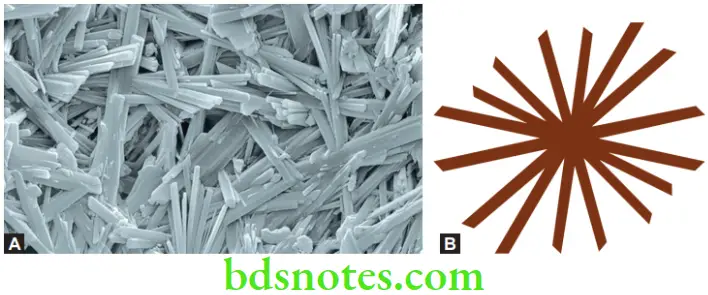
- Microporosity resulting from the growth of gypsum crystals. These voids are associated with setting expansion and are smaller than the first type. They appear as angular spaces between individual crystals in the aggregate.
Manipulation
Proportioning
- To secure maximum strength a low water/powder ratio should be used. The water should be measured and the powder weighed.
Water/powder ratio
- The W/P ratio is a very important factor in deciding the physical and chemical properties of the final product.
- For example The higher the water-powder ratio, the longer the setting time and the weaker will be the gypsum product. Therefore, the water or powder ratio should be kept as low as possible but at the same time sufficient to produce a workable mix.
The water requirement of a product is affected by
- Shape and compactness of crystals Thus, irregular, spongy plaster particles need more water than denser stone.
- Small amounts of surface active materials like gum arabic plus lime markedly reduce the water requirement of all gypsum products.
- Particle size distribution Grinding of the powder breaks up needle-like crystals. This improves packing characteristics and reduces the water needed.
Recommended w/p ratio
Impression plaster: 0.50 to 0.75
Dental plaster: 0.45 to 0.50
Dental stone: 0.28 to 0.30
Die stone, Type 4: 0.22 to 0.24
Die stone, Type 5: 0.18 to 0.22
Instruments
Flexible rubber/plastic bowl, stiff-bladed spatula.
Procedure For Hand-Mixing
- Water is taken first to prevent adherence of dry powder to the sides of the bowl. Water and powder are dispensed according to the recommended W/P ratio.
- The powder is sifted into the water in the rubber bowl. Plaster/stone dispensers are also available.
- It is allowed to settle for 30 seconds to minimize air entrapment.
- The mix is stirred vigorously. Periodically wipe the inside of the bowl with a spatula to ensure wetting of the powder and breaking up of lumps.
- Continue till a smooth creamy mix is obtained. Spatulation should be completed in 45 to 60 seconds.
- Vibrate the mix (using a mechanical vibrator or by repeated tapping against a bench) and pour it into the impression, taking care not to entrap the air.
The mixing equipment must be meticulously clean. There should be no particles of set plaster from a previous mix sticking to the bowl or spatula.
These if present will act as additional nuclei of crystallization and cause faster setting. No air must be trapped in the mixed mass. It causes a loss of surface detail and weakens the cast.
Mechanical Mixing
Mechanical mixing under vacuum gives stronger and denser casts. However, the equipment is expensive.
Setting Time
- The time elapsing from the beginning of mixing until the material hardens is called setting time.
- Mixing time It is the time from the addition of the powder to the water until mixing is complete. A mixing time of 1 minute is usually sufficient.
- Working time Itis the time available to work with the mix for the intended purpose, i.e., one that maintains an even consistency.
- At the end of the working period, the material thickens and is no longer workable. The freshly mixed mass is semifluid in consistency and quite free-flowing. A working time of 3 minutes is usually sufficient.



- Initial setting time As the reaction proceeds, more hemihydrate crystals react to form dihydrate crystals. The viscosity of the mass is increased and it can no longer be poured. The material becomes rigid (but not hard). It can be carved but not moulded. This is known as initial setting time.
- Final setting time The time at which the material can be separated from the impression without distortion or fracture.
Measurement of setting time
Usually by some type of penetration test. Occasionally, other tests are used.
- Loss of gloss method As the reaction proceeds the gloss disappears from the surface of the plaster mix (sometimes used to indicate the initial set).
- Exothermic reaction The temperature rise of the mass may also be used for the measurement of setting time as the setting reaction is exothermic.
- Penetration tests By using penetrometers.
Types of penetrometers
- Vicat needle
- Gillmore needles
Vicat needle It weighs 300 gm and the needle diameter is 1 mm. The time elapsing from the start of mixing till the needle does not penetrate to the bottom of the plaster is the setting time. The setting time obtained with the Vicat needle is similar to the initial Gillmore.
Gillmore needles Two types—small and large. The small Gillmore needle has a 1/4 lb weight and a diameter of 1/12” (2.12 mm) while the large Gillmore has a 1 lb wt and diameter of 1/24” (1.06 mm).
Initial Gillmore The time elapsing from the start of mixing until the time when the point of the 1/4 lb Gillmore needle no longer penetrates the surface is the initial setting time


Final Gillmore Similarly the time elapsing from the start of mixing until the point of the 1 lb Gillmore needle leaves only a barely visible mark on the surface of the set plaster is known as the final setting time.
Factors affecting setting time
- Manufacturing process
- Mixing and speculation (time and rate)
- Water/Powder ratio
- Temperature
- Modifiers
Manufacturing process
- If calcination is incomplete and excess gypsum (dihydrate) is left in the final product, the resulting plaster will set faster.
- If soluble anhydrite is in excess, the plaster will set faster.
- If natural anhydrite is in excess, the plaster will set slowly.
- Fineness Finer the hemihydrate particle size, the faster the set, because
- Hemihydrate dissolves faster, and
- The gypsum nuclei are more numerous and therefore, crystallization is faster.
Mixing and spatulation Within limits the longer and faster the plaster is mixed, the faster it will set because nuclei of crystallization are broken and well-distributed within the mass.
- Water/Powder ratio The More water used for mixing, the fewer the nuclei per unit volume. Thus setting time will be prolonged.
- Temperature On increasing from a room temperature of 20 °C to a body temperature of 37 °C, the rate of the reaction increases slightly and the setting time is shortened.
- As the temperature is raised above 37 °C the rate of reaction decreases and the setting time is lengthened.
- At 100 °C the solubilities of hemihydrate and dihydrate are equal, in which case no reaction can occur and the gypsum will not be set.
- Modifiers (Accelerators and Retarders) Modifiers are chemicals added to alter some of the properties and make them more acceptable to the dentist.
If the chemical added decreases the setting time it is called an accelerator, whereas if it increases the setting time it is called a retarder.
Accelerators
- Finely powdered gypsum (up to 1%) is added by manufacturers to accelerate setting time. Acts by providing additional nuclei of crystallization. One source of gypsum is slurry water.
- In low concentrations, salts like sodium or potassium sulfate (2 to 3%) and sodium chloride (up to 2%) are accelerators. They act by making the hemihydrate more soluble.
Retarders
Retarders generally act by forming a layer on the hemihydrate to reduce its solubility. It also inhibits the growth of gypsum crystals.
- Borax (1–2%) is the most effective retarder. During setting, it forms a coating of calcium borate around the hemihydrate. Thus, the water cannot come in contact with the hemihydrate.
- In higher concentrations, sodium chloride (3.4% to 20%) and sodium sulfate act as retarders. In higher concentrations, the salt precipitates and poisons the nuclei of crystallization.
- Acetates, borates, citrates, tartrates, and salts like ferric sulfate, chromic sulfate, aluminum sulfate, etc., are retarders, which act by nuclei poisoning by reducing the rate of solution of hemihydrate or by inhibiting the growth of dihydrate crystals.
- Some additives react with hemihydrate, e.g., soluble tartrates and citrates precipitate calcium tartrate and citrate, respectively.
- Colloids such as gelatin, glue, agar, coagulated blood, etc. are effective retarders, presumably acting by nuclei poisoning.
- Contact with the gypsum during setting results in a soft, easily abraded surface. To avoid The impression should be thoroughly rinsed in cold water to remove blood and saliva before pouring.
Properties
The important properties of gypsum products are
- Setting expansion
- Strength
- Hardness and abrasion resistance
- Reproduction of detail
Setting Expansion
Setting expansion is measured using an extensometer. Setting expansion is of two types
- Normal setting expansion
- Hygroscopic setting expansion
Normal Setting Expansion (0.05 to 0.5%)
All gypsum products show a linear expansion during setting, due to the outward thrust of the growing crystals during setting. Crystals growing from the nuclei not only intermesh but also intercept each other during growth.
Importance of setting expansion In dentistry, setting expansion may be both desirable and undesirable depending on the use. It is undesirable in impression plaster, dental plaster, and stone as it will result in an inaccurate cast or change in the occlusal relation if used for mounting.
Increased setting expansion is desired in the case of investment materials as it helps to compensate for the shrinkage of the metal during casting.
Control of setting expansion
- Mechanical mixing reduces setting expansion when compared to hand-mixed stone.
- An increase in the W/P ratio reduces the setting expansion.
- Modifiers generally reduce the setting expansion.
- Potassium sulfate 4% solution reduces setting expansion from 0.5 to 0.06 %.
- Sodium chloride and borax also decrease setting expansion.
For accuracy in dental procedures, the setting expansion has to be minimized. The manufacturers achieve this by the addition of K2SO4.
This, however, reduces the setting time. To counteract this, retarders like borax are also added (borax also reduces setting expansion).
Hygroscopic Setting Expansion
When a gypsum product is placed under water before the initial set stage, a greater expansion is seen. This is due to hygroscopic expansion. When expansion begins, externally available water is drawn into pores forming in the setting mass and this maintains a continuous aqueous phase in which crystal growth takes place freely.
Under dry conditions, this additional water is not available and as expansion occurs, the aqueous phase in the mix is reduced to a film over the growing crystals. It is greater in magnitude than normal setting expansion.
Importance Used to expand some gypsum bonded investments.
Strength
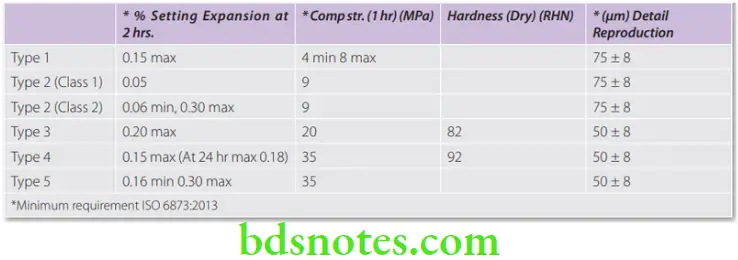
The strength increases rapidly as the material hardens after the initial setting. Minimum strength requirements (ISO) for various gypsum products
Factors affecting strength
The free water content (excess water) The greater the amount of free water in the set stone, the less the strength.
Wet strength It is the strength when excess free water (more than is necessary for reaction) is present in the set gypsum. The wet strength (1-hour compressive strength) for model plaster, dental stone, and die stone are 12.5, 31 and 45 MPa respectively.
Dry strength It is the strength of gypsum when the excess free water is lost due to evaporation. It is two or more times greater than the wet strength.
Excess water may be removed from gypsum cast by low-temperature drying. But there is no strength increase until the last 2% of free water is removed.
This strength increase on drying is reversible, thus soaking a dry cast in water reduces its strength to the original level. Many products have strength values over the ISO requirements. One Type 4 product claims a wet strength (1 hr) of 67 MPa and a dry strength of 121.6 MPa.
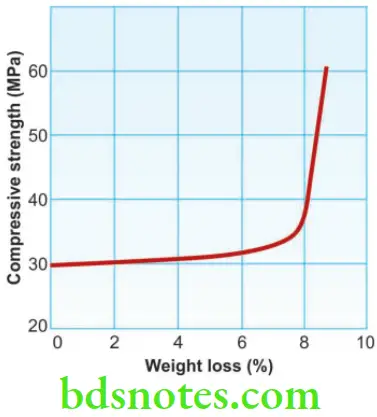
Temperature Gypsum is stable only below about 40 °C. Drying at higher temperatures must be carefully controlled. Loss of water of crystallization occurs rapidly at 100 °C or higher and causes shrinkage and a reduction in strength.
Other factors affecting strength
- W/P ratio The more water, the greater the porosity and less the strength.
- Spatulation Within limits, strength increases with increased spatulation.
- Addition of accelerators and retarders Lowers strength.
Tensile Strength
Gypsum is a brittle material, thus weaker in tension than in compression.
- The one-hour tensile strength of model plaster is approximately 2.3 MPa. When dry, the tensile strength doubles. The tensile strength of dental stone is twice that of plaster.
- Significance Teeth on a cast may fracture while separating from the impression. Since in practice fracture of gypsum typically occurs in tension, tensile strength is a better guide to fracture resistance.
- Time at which cast can be used The cast cannot be used as soon as it reaches its final setting (as defined by the Vicat and Gillmore tests). This is because the cast has not reached its full strength. T
- echnically the cast can be used when it has attained at least 80% of its one-hour strength. Current products are ready for use in 30 minutes.
Hardness and Abrasion Resistance
- Dies and casts are often used to construct restorations and prostheses. A good surface hardness and abrasion resistance is therefore essential.
- Hardness is related to the compressive strength. The higher the compressive strength of the hardening mass, the higher the surface hardness. After the final setting occurs, the surface hardness remains practically constant until most of the excess water is dried, after which it increases.
- The surface hardness increases at a faster rate than the compressive strength since the surface of the hardened mass reaches a dry state earlier than the inner portion of the mass. Commercial hardening solutions are available to increase the surface hardness of stone. However, surface hardness and abrasion resistance are not always related, for example, epoxy resin is more abrasion resistant than die stone, even though die stone is harder of the two.
Flow
- The flow of freshly mixed gypsum depends on the amount of water used (W/P ratio). The greater the amount of water used, the greater would be the flow.
- However, a correctly proportioned mix has sufficient flow. Vibrating the mix greatly improves the flow. The flow reduces as it approaches its initial set.
Reproduction of Detail
Gypsum products reproduce detail accurately.
Significance
- Impression plaster has to accurately record oral tissues.
- Cast material has to duplicate all the detail recorded by the impression.
Factors which affect detail reproduction include compatibility with the impression material, trapped air bubbles in the mix and surface contaminants like saliva. Use of a mechanical vibrator and proper technique considerably improve detail reproduction.
Specialized Gypsum Products
Some gypsum products are manufactured for specific uses in dentistry. Each type is developed with specific physical properties suitable for the particular purpose.
Dental Casting Investments
Uses
To prepare refractory molds for casting dental alloys.
Adding a refractory material like silica or quartz or cristobalite to dental plaster or stone permits it to withstand high temperatures. These are called dental casting investments (detailed in Chapter on investments).

Divestment
Uses
To make refractory dies.
- It is a combination of die stone and gypsum-bonded investment mixed with colloidal silica. A die is made and the wax pattern constructed on it. Then the entire assembly (die and pattern) is invested in the divestment (normally the wax pattern is removed from the die and invested separately).
- The setting expansion of the material is 0.9% and thermal expansion is 0.6% when heated to 677 °C. The advantage of divestment is that the wax pattern does not have to be removed from the die, thus distortion of the pattern can be avoided.
Synthetic Gypsum
- It is possible to make alpha and beta hemihydrate from the byproducts during the manufacture of phosphoric acid.
- The synthetic product is usually more expensive than that made from natural gypsum, but when the product is properly made, its properties are equal to or exceed the latter. However, manufacture is difficult and a few have succeeded (e.g., Japan and Germany).
Orthodontic stone
- For orthodontic study models, many orthodontists prefer to used white stone or plaster. These products have a longer working time for pouring of multiple models. To produce a glossy surface, finished models may be treated with ‘model glow’ model soap.
Resin modified stones
- A new resin fortified die stone (e.g., ResinRock, Whipmix corporation) is available. It is a blend of synthetic resin and alpha gypsum. These stones are less brittle, have improved surface smoothness and increased resistance to abrasion. When mixed, it forms a creamy, thixotropic mix which flows more easily under vibration. Their compressive strength can be as high as 79 MPa.
Mounting plaster
- Plaster used for attaching the cast to the articulator is known as mounting plaster. Regular plaster (type 2, class 1) has higher setting expansion and should be avoided for mounting. However, plasters with lower setting expansion (described by ISO 6873:2013 as Type II, Class 1) specialized for this purpose are available commercially.
- Important properties for these products include a low setting expansion (0 to 0.05 %) which is important for the accuracy of the mounting, low strength (12 MPa) which allows easy separation from the cast and fast setting time (3 minutes).
Fast Setting Stone
- These are exceptionally fast-setting stones (2 minutes) with an early high compressive strength (1 hour – 41 MPa) which allows separation of the cast from the impression in 5 minutes. An example includes Snap stone (Whipmix).
Care of Gypsum
Care of the Cast
If the gypsum cast has to be soaked in water it must be placed in a water bath in which plaster debris is allowed to remain constantly on the bottom of the container to provide a saturated solution of calcium sulfate at all times. This is known as ‘slurry water’.
If the cast is washed in ordinary water, the surface layer may dissolve, hence slurry water is used to preserve surface details. Such a procedure also causes a negligible expansion.
All gypsum casts must be handled carefully as any departure from the expected accuracy may result in a poorly fitting appliance.
Storage of the Powder
- As plaster is hygroscopic, it should be kept in air-tight containers. When the relative humidity is more than 70%, plaster starts taking up moisture initiating a setting reaction. This produces small crystals of gypsum which act as nuclei of crystallization. Thus in the early stages, moisture-contaminated plaster sets faster.
- In later stages, as the hygroscopic action continues, the entire hemihydrate mass is covered by more crystals of gypsum. The water penetrates the mass with difficulty, thereby delaying the setting.
- Thus heavily moisture contaminated stone or plaster sets slower. The humidity factor is a major consideration in parts of India with high atmospheric humidity.

- It should be kept clean with no dirt or other foreign bodies.
Infection Control
There has been an increased interest over possible cross-contamination to dental office personnel through dental impressions. If an impression has not been disinfected, it is wise to disinfect the stone cast.
Gypsum products may be disinfected by
- Immersing cast in a disinfection solution.
- Addition of disinfectant into the stone.
- Overnight gas sterilization while treating patients known to have an infection (impractical for routine use).
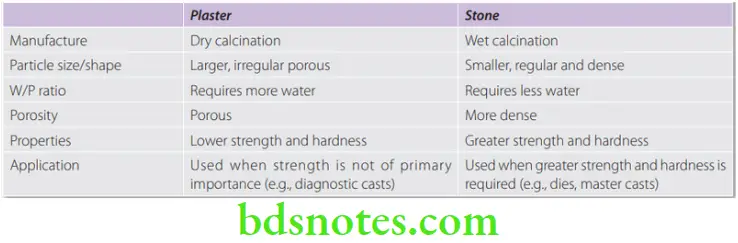
Differences between Dental Plaster and Dental Stone

Leave a Reply