Phenotypic Expression Of Gene
Gene Expression
The process by which the biological information contained in the gene is made available to the cell is called gene expression.
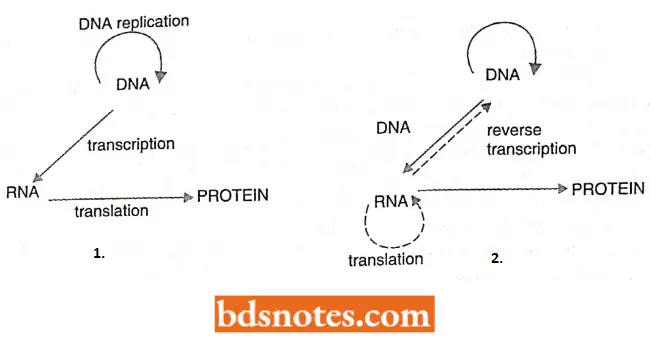
The Central Dogma: The process that we know as gene expression was put forward by Francis Crick in 1958 in a lecture called “On protein synthesis” presented to the Society For Experimental Biology.
- Crick postulated that the biological information contained in the DNA of the gene is transferred first to RNA and then to protein.
- Crick also stated that the information flow is unidirectional, that proteins cannot themselves direct synthesis of RNA, and that RNA cannot direct synthesis of DNA (In other words, once information has passed to protein it cannot get out again).
- This part of central dogma was shaken in 1970 when Howard Temin and David Baltimore independently discovered that certain viruses do transfer biological information from RNA to DNA.
- However, the central dogma remains today as one of the underlying concepts of molecular genetics.
Gene Expression Occurs In The Following Steps
Transcription (The first stage of gene expression). All genes undergo the first stage of gene expression which is called transcription.
- During transcription, the template strand of the gene directs the synthesis of an RNA molecule, the sequence of this RNA transcript being DNA determined by complementary base pairing.
Translation (The second stage of gene transcription expression). For some genes, the RNA transcript is itself the end-product of gene expression.
- For others, the transcript undergoes the second stage of gene expression, called translation.
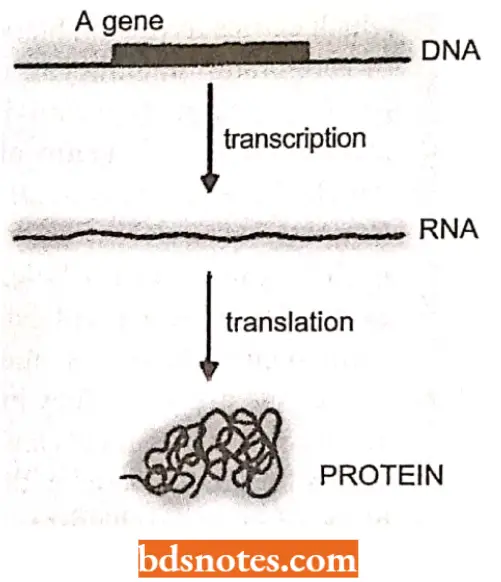
- During translation, the RNA molecule (called messenger RNA or mRNA) directs the synthesis of a polypeptide, the amino acid sequence of which is determined by the nucleotide sequence of the mRNA ( which is of course itself derived from the nucleotide sequence of the gene).
- Each triplet of adjacent ribonucleotides specifies a single amino acid of the polypeptide, the identity of the amino acid corresponding to each triplet being set by the genetic code.
Protein synthesis (Key to the expression of biological information). For all genes, the end-point of gene expression is a synthesis of either an RNA molecule or a polypeptide.
- How can this simple information bring about the utilization of the biological information contained in genes and thereby enable the construction (building) of a living organism
- The answer lies in the functional flexibility of protein molecules. This is because polypeptides of different amino acid sequences can have quite different chemical properties and can therefore play quite different roles in the cell as follows:

Structural proteins. They form parts of the framework of organisms. Examples are collagen which is associated with tendons, bone, and cartilage in vertebrates, sclerotin in the exoskeleton of insects, and virus-coat proteins.
Contractile proteins. They enable organisms to move. Examples are actin and myosin in muscles, and dynein in cilia and flagella.
Enzymes. They catalyze the multitude of biochemical reactions that bring about the release and storage of energy (catabolism) and the synthesis of new compounds ( anabolism).
- Examples are hexokinase, the enzyme that catalyzes the first step in glycolysis; RNA polymerase, which is responsible for the transcription of DNA into RNA during gene expression; tryptophan synthetase, which catalyzes the last step in the lengthy biosynthetic pathway that converts phosphoenol- pyruvate into the amino acid tryptophan.
Transport proteins. They carry important molecules around the body.
- Examples are hemoglobin, which carries oxygen in the bloodstream of vertebrates, haemocyanin, which performs the same function in some invertebrates; and serum albumin, which transports fatty acids in the blood.
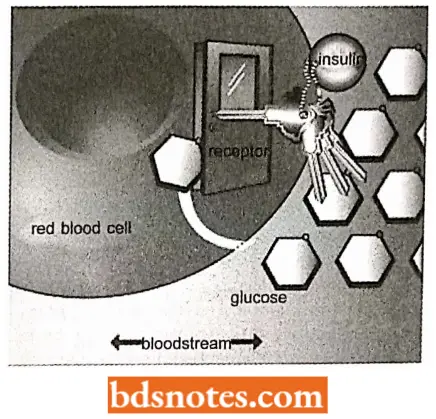
Regulatory proteins. They control and coordinate biochemical reactions in the cell and the organism as a whole.
- Examples are catabolic activator protein, which regulates the expression of genes involved in sugar metabolism in E.coli, and hormones such as insulin, which controls glucose metabolism in vertebrates.
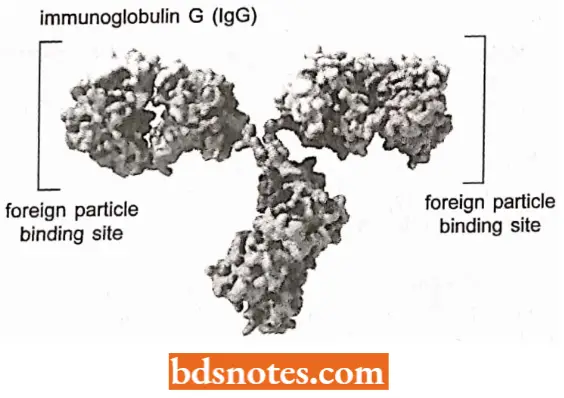
Protective proteins. They have evolved to the greatest sophistication in vertebrates and protect against infectious agents and injury. Examples are immunoglobulins and other antibodies which form complexes with foreign proteins, and thrombin and other components of the blood clotting mechanism.
Storage proteins. They store compounds and molecules for future use by the organism. Examples are ovalbumin, which stores amino acids in egg white, and ferritin, which stores iron in the liver.
- The development and functioning of a living organism can be looked at as the coordinated activity of a vast range of different protein molecules.
- The biological information obtained in the genes, the blueprint for life itself, is simply the instructions for synthesizing these proteins at the correct time and in the correct place.

Phenogenesis
- DNA of genes has two essential functions :
- Replication or self-reproduction and Intervention in phenogenesis.
Lipogenesis is a mechanism by which the phenotype of an organism is realized or produced under the control of DNA in a given environment which includes not only external factors such as temperature and the amount or quality of light, but also internal factors such as hormones and enzymes.
- The phenotype of an organism is not the direct outcome of the action of its DNA but is the result of various embryological and biochemical activities of its cells from the zygotic to the multicellular stage.
- All these cellular activities involve a variety of structural and functional or enzymatic proteins. Enzymatic proteins or enzymes perform catalytic functions, causing the splitting or union of various cellular molecules.
- All of the biochemical reactions of cells constitute the subject of intermediary metabolism. Each reaction of intermediary metabolism occurs as stepwise conversions of one substance into another.
- All the steps that transform a precursor substance to its end product which is ultimately expressed into a structural or functional phenotypic trait, constitute a biosynthetic pathway.
- Each step of a biosynthetic pathway is catalyzed by a specific enzyme which in its turn being produced by a specific gene.
It has become clear now that
- DNA of genes itself does not have an enzymatic character and, therefore, does not involve directly in a biosynthetic pathway of cell;
- Between the gene and final product or between the gene and enzyme there is a long, tortuous path;
- The immediate or primary gene product is a kind of RNA called mRNA (messenger ribonucleic acid) which is complementary to the DNA of the genes and controls the assemblage of the amino acids to form enzymes, at the surface of cytoplasmic ribosomes.
Thus, to produce a particular phenotypic trait DNA transcribes mRNA which translates into an enzymatic or structural protein which ultimately produces a phenotypic trait by the process of phenogenesis. The relationship between genes and enzymes had been suspected very early in the history of biochemical or physiological genetics.
All Enzymes Are Not Proteins: For several decades, the dogma in biology has been that molecular reactions occurring in living cells are catalyzed by enzymes that are composed of polypeptides.
- We now know that the introns of some precursor RNA molecules such as the rRNA precursors in Telrahymena thermophilia are removed autocatalytically (“self-spliced”) with no involvement of any protein (Kruger et al., 1982).
- Some primary transcripts of genes in mitochondria and chloroplasts are also excised autocatalytically. An RNA molecule that has catalytic activity is called a ribozyme.
- Introns are intervening sequences, of DNA bases within eukaryotic genes that are not represented in the mature RNA transcripts because they spliced out of the primary RNA transcript.
Gene-Enzyme Relation
The understanding of correlations between genes and the phenotype of an organism requires a consideration of the consequences of gene malfunction since the expression of abnormal gene effect is a major source of the information gained concerning normal gene function.
- The groundwork for a functional relationship between genes and enzymes was laid in 1902 when Bateson reported that a rare human defect alcaptonuria was inherited as a recessive trait.
- Then in 1909, Garrod, ‘in his book, “Inborn Errors of Metabolism” suggested a relationship between genes and specific chemical reactions.
- Alcaptonuria was among the heritable diseases with which he dealt at length. But his remarkable piece of work remained unnoticed until 1940 when Beadle, Tatum, Ephrussi, and other geneticists had got modem understanding of gene action by performing experiments on Ncumspora and Drosophila.
- They found that mutational changes in genes can be related to losses of specific enzymes.
- This concept was widely known as the “one gene one enzyme hypothesis.”
- This hypothesis can be understood well by knowing the phenomenon of metabolic block.
Metabolic Block
A biosynthetic pathway, often, requires more than two enzymes, and each enzyme is specified by a single gene. If there occurs any mutation in any gene, then either the production of normal enzyme will be ceased or a defective enzyme will be produced.
- This defective enzyme instead of determining the normal phenotype, will determine an abnormal, defective, or mutant phenotype. Such mutant genes, which alter the enzymes of a biosynthetic pathway in such a manner that a defective phenotype results are said to cause “metabolic blocks”.
- The metabolic blocks have been found to cause various abnormal phenotypes or diseases in a variety of organisms and provide the best opportunities to understand the relation of genes with enzymes and phenotypes of an organism more properly.
- Thus, the mutations in genes that control sequential biological steps have proved to be in\aluablc for “dissecting” the number and order of reactions in a given biosynthetic pathway.
- In particular, a field of study known as biochemical genetics has combined genetics and biochemistry to elucidate the nature of metabolic pathways, most notably in haploid organisms whose growth requirements are known and whose gene expression is not complicated by allelic interactions.
Approach Of Biochemical Genetics: The approach followed in biochemical genetics is to assemble a collection of mutant strains that cannot synthesize a particular component and thus require it for growth.
- These strains are subjected to complementation tests to estimate how many separate genes are involved, and the genes are mapped to determine their relationships.
- Strains in a given complementation group are then tested for their ability to grow when supplied with known metabolic precursors of the final component.
- It then can grow in the presence of a certain precursor, they must suffer from a genetic lesion affecting a step before the synthesis of that precursor.
- If instead, they cannot grow when a certain precursor is supplied, the genetic lesion must affect a step that follows the synthesis of that precursor.
Examples Of Biochemical Genetics
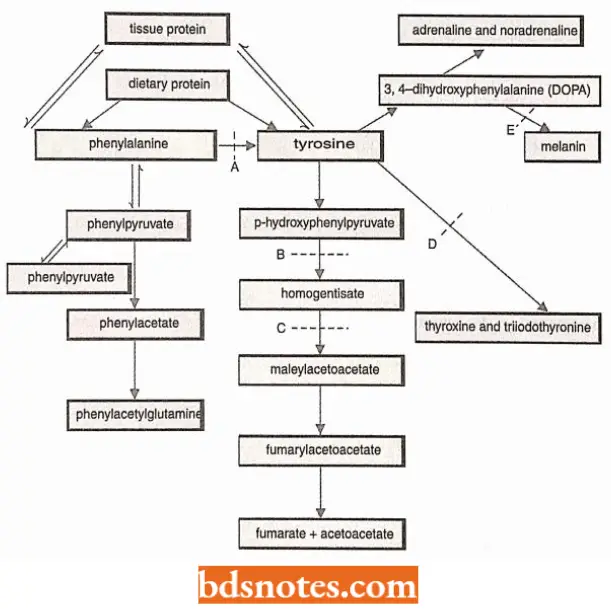
Phenylalanine Metabolism in Human Beings The metabolism of the aromatic amino acids—phenylalanine and tyrosine in human beings provides the best example of a gene-controlled, enzyme-catalyzed biochemical reaction.
- In human beings, phenylalanine is an essential amino acid that must be supplied in the dietary proteins. Once in the body, phenylalanine may follow any of three paths.
It may be
- Incorporated into cellular proteins,
- Converted to phenyl pyruvic acid, or
- Converted to tyrosine.
The conversion of phenylalanine into tyrosine takes place in the presence of phenylalanine hydroxylase enzyme, in the liver cells.
- Tyrosine is converted in turn to 3-4-dihydroxy phenylalanine (nick-named DOPA) by another enzyme and DOPA serves as a precursor for the hormones adrenaline and noradrenaline and the black pigment, melanin.
- Tyrosine itself serves as a precursor of the hormones thyroxine and triiodothyronine. Excess tyrosine is degraded to carbon dioxide and water by a series of steps that involve the formation of p-hydroxyphenyl pyruvate, 2-5 dihydroxy phenylpyruvate, homogentisic acid, maleylacetoacetic acid, fumaric acetoacetic acid, and fumaric and acetoacetic acid.
- Excess phenylalanine is degraded by a series of steps to compounds which include phenyl pyruvic acid and phenylacetic acid.
Genetic Disorders Of The Phenylalanine Metabolism And Resulting Diseases. Five rare diseases in human beings result from the improper functioning of five enzyme systems (i.e., metabolic blocks) involved in the metabolism of phenylalanine, tyrosine, and their derivatives.
- All of these diseases are due to mutant, autosomal recessive genes in homozygous conditions. These diseases are the following:
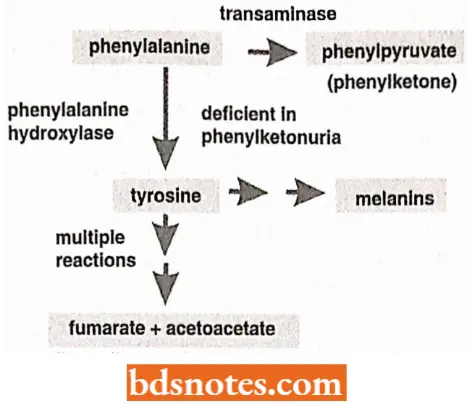
Phenylketonuria. Persons with genotype pp fail to produce the enzyme phenylalanine hydroxylase (para hydroxylase) with the result that phenylalanine fails to convert into tyrosine and consequently, the concentration of phenylalanine rises in the blood plasma, cerebrospinal fluid, and urine.
- The urine of phenylketonuria (PKU) patients contains (in addition to phenylalanine) elevated amounts of phenyl pyruvic acid, phenyl lactic acid, and other derivatives of phenylalanine.
- PKU patients have light pigmentation and are physically and mentally retarded.
The feeble-mindedness in PKU patients is thought to be due to an impairment of the brain tissues by the phenylpyruvic acid in the cerebrospinal fluid.
Alkaptonuria. The persons with genotype hh fail to produce the en/.yme homogentisic acid oxidase which catalyzes the oxidation of homogentisic acid.
- Therefore, in them, normal oxidation of homogentisic acid into water and carbon dioxide does not occur and large amounts of homogentisic acid are excreted in the urine, which turns black upon exposure to the air.
- Moreover, the homogentisic acid accumulates in the body and becomes attached to the collagen of cartilage and other connective tissues, due to which, the ear and sclerae are stained black. Persons with such phenotypic abnormalities are said to have alkaptonuria disease.
Tyrosinosis. The recessive gene, t in its homozygous condition, blocks the conversion of p-hydroxyphenylpyruvate into 2, 5-dihydroxyphenyI pyruvate.
- This leads to the accumulation of tyrosine, excesses of which are excreted via urine. This condition is called tyrosinosis. It is reported in only one human and causes no harmful effect.
Goitrous cretinism. The persons with the cc genotype fail to produce the enzyme that is required for the conversion of tyrosine into thyroxine and triiodothyronine hormones in their thyroid glands.
- This condition is called goitrous cretinism which is accompanied by a considerable degree of physical and mental retardation and hypertrophy of the thyroid gland.
Albinism. The persons with recessive aa 1 genotype lack the tyrosinase enzyme system which is required for the conversion of 3, 4- dihydroxyphenyl alanine (DOPA) into melanin pigment inside the melanocytes.
- In an albino patient, melanocytes are present in normal numbers in their skin, hairs, iris, etc., but lack in melanin pigment.

Eye Pigmentation in Drosophila The metabolism of Drosophila eye pigment (ommochrome) xanthomata suggests a gene enzyme-relationship.
- The dull red compound eyes of wild-type Drosophila are produced by deposition of brown pigment (xanthomatous) in the periphery of each ommatidium and red pigment (drosopterins) in the center of each ommatidium of the compound eye.
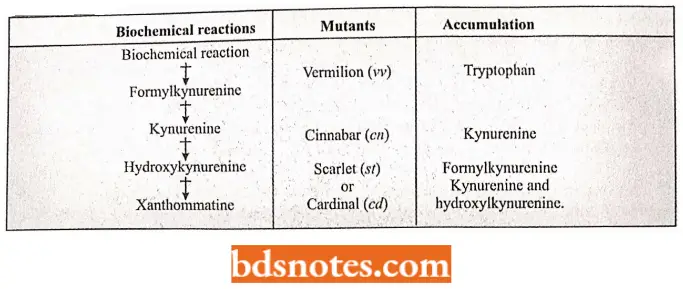
- The xanthommatins are the end products of the metabolism of the amino acid tryptophan which is taken by Drosophila along with food as an essential amino acid.
- To form xanthomata, tryptophan first is converted into formyl kynurenine, which converts into kynurenine, and the kynurenine by undergoing via hydroxykynurenine compound forms xanthomata.
- Four recessive nonallelic genes are known which block the synthesis of the xanthommatin but which have no effect upon the formation of the drosopterins. As a consequence, all the mutant insects are characterized by compound eyes which have a bright red color.
- Individuals homozygous for the sex-linked gene vermilion (v) fail to convert tryptophan into formylkynurenine and so accumulate tryptophan.
- Individuals homozygous for the second chromosomal gene cinnabar (cn) cannot convert kynurenine into hydroxykynurenine and as a consequence, they accumulate kynurenine.
- Individuals homozygous for the third chromosomal mutants scarlet (st) or cardinal (cd) produce the water-soluble compounds, formylkynurenine, kynurenine, and hydroxykynurenine.
Gene Control Of Tryptophan Metabolism In Drosophila:
The Arginine Pathway In Neurospora: The concept of one gene-one enzyme-one phenotypic effect relationship, is best exemplified by Neurospora crassa.
- The wild-type Neurospora crassa can live on a simple artificial medium containing inorganic salts, a source of organic carbon, and the vitamin biotin (such simple medium is called a minimal medium).
- It has the inborn capacity to synthesize all the other vitamins, amino acids, and nitrogenous bases essential to normal development.
- On the assumption that this capacity was genetically conferred, Beadle and Tatum argued that mutation could lead to nutritional requirements over and above those satisfied by a minimal medium and that the nature of the biosynthetic (metabolic) block could be determined from the nature of the growth supplement which restored normal development.
- They found that the treatment of conidia of N.crassa by X-rays or ultra-violet rays increased the frequency of such mutant strains which could not survive on minimal medium but developed normally when one or other supplement was added to it.
- In each instance, genetic tests show that the mutant strain produced by irradiation differs from the normal wild type by a single gene, and chemical tests show that the addition of a single substance to the minimal medium will enable the mutant strain to grow normally.
The Arginine Pathway In Neurospora:
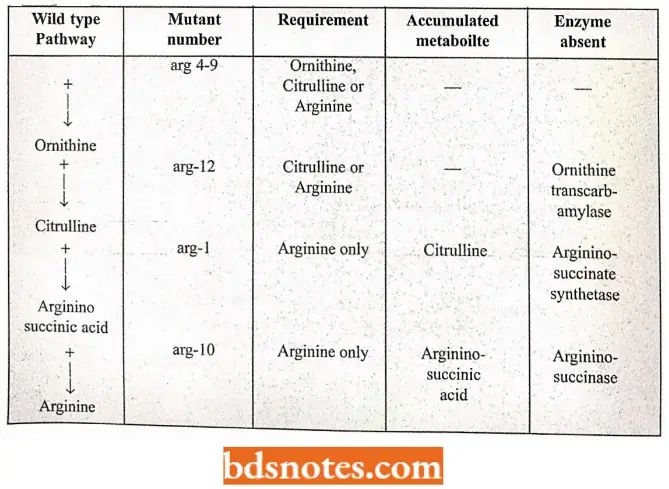
- This indicates that each normal gene of the wild strain produces a single enzyme that regulates one step in the biosynthesis of this particular chemical; the mutant gene does not produce this enzyme. For example, wild-type N.crassa can synthesize arginine amino acids such as follows:-
Sugar + ammonia (in minimal medium)— → glutamic acid — → glutamic semialdehyde — → Ormithin— → citruline — → arginine.
- But there are ornithine about 12 mutant citrulline strains of N arginine. crassa which loses the capacity to synthesize arginine. These mutant strains are called arginine mutant strains and are numbered arg-1, arg-2……arg-12.
- It has been found that out of these 12 arginine mutants, some strains (arg-1 and arg- 10) have specific requirements for arginine only; other strains (for example., arg-1 2) grow normally if either arginine or citrulline is added to the environment; still, different strains (for example., arg-4 to arg-9) can grow on a supplement of arginine, citrulline or ornithine.
- The existence of these three types of mutants indicates a sequence of reactions involved in the synthesis of arginine.
- Since some mutants can utilize citrulline but not ornithine, the latter must precede citrulline in the arginine synthetic pathway. Similarly, \citrulline must precede arginine.
- Some of the steps of the arginine pathway as exemplified by these three kinds of mutants of N. crassa have been summarized.
Arginine Biosynthesis Pathway of E. coli: Certain mutant strains of E. coli require arginine for growth. Biochemical genetics of these strains of E. coli have shown that the arginine biosynthesis pathway of E. coli involves eight genes and strains having a mutation in any one gene from these eight contain specific growth requirements.
- For example, arg H strains can grow only if they are given arginine; no other precursor will do.
- The arg G strains, on the other hand, will grow either in the presence of arginine or arginosuccinic acid.
- The arg F strains can grow if supplied with arginine, arginosuccinic acid citrulline, and so on.
- The arginine biosynthetic pathway of E. coli can be represented as follows:
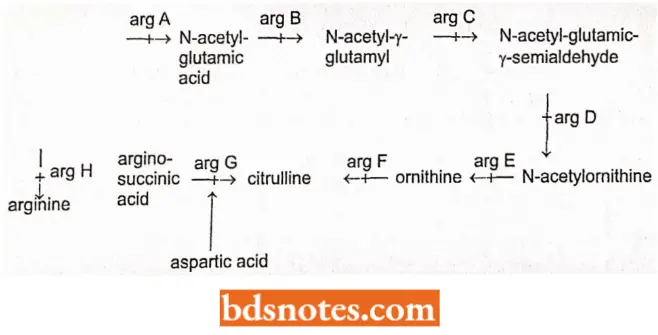
Besides, Neurospora, E. coli, Lactobacillus (Volcani and Snell, 1948) and Aspergillus (Pontecorvo 1953) have similar arginine pathways and so have confirmed the concept of one geneone enzyme-one phenotype concept more strongly.
Genetic Dissections Of Complex Biochemical Pathways
The approach of biochemical genetics has later on used to dissect quite complex metabolic pathways of certain microbes (bacteriophages and Chlamydomonas).
Perhaps the most elegant of these studies, initiated by R. Edgar and W. Wood, concerns the morphogenesis of the phage T4, a process that involves the elaborate and sequential self-assembly of numerous different kinds of proteins.
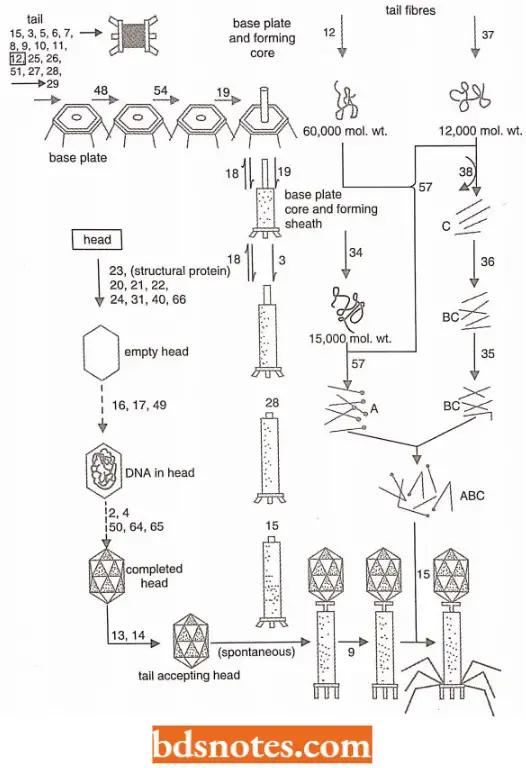
- The analysis begins by isolating conditional lethal strains of T4 bacteriophage that carry amber or temperature-sensitive mutations in genes essential to the assembly of mature phages.
- Each strain is allowed to infect E. coli cells under non-permissive conditions, (Note: Permissive means supporting genetic replication as of a virus).
- Whereupon phage assembly proceeds up to the point at which an essential protein is missing or inactive. Depending on the absent protein, different precursor phage structures will accumulate in the infected cells.
- If the cells are now lysed artificially, the precursor structures can often be isolated and observed with the electron microscope and can also be subjected to in vitro complementation tests with precursor structures derived from other lysates. The complementation patterns of the various strains can also be studied in vivo.
- In this way, some 50 genes have been found to be involved in T4 phage morphogenetic processes, either by directly specifying some structural protein of the phage coat or by specifying a protein required in smaller amounts for some delicate assembly process.
- The sequence of events involved in the morphogenesis of T4 bacteriophage and the relevant genes, as they are presently.
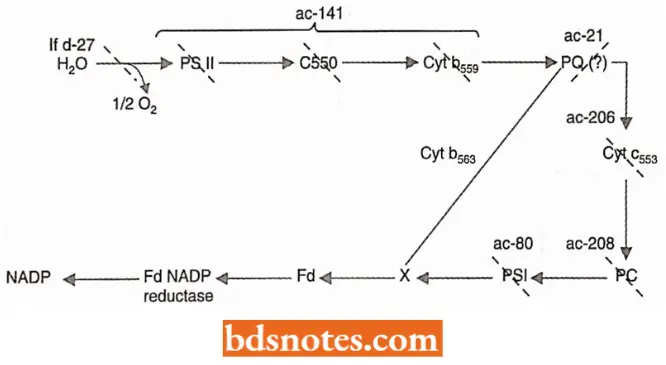
Mutant strains of Chlamydomonas Reinhardt have been similarly used to elucidate the sequence of events in photosynthetic electron transport. Such mutant strains cannot carry out photosynthesis and thus require acetate (ac) for growth.
- Analysis of broken cell preparations reveals that the chloroplast membranes do not possess an intact photosynthetic electron transport chain.
- The normal chain constitutes a series of molecules that become alternately reduced and oxidized as electrons derived from water flow from one to the next and finally reduce NADP, the terminal electron acceptor.
- If a component in the chain is missing or inactive as a consequence of mutation, electron flow will proceed to the point and stop.
- Such strains, however, are often able to carry out partial reactions in vitro, in which artificial electron donors and/or acceptors are provided to accept or donate electrons in specific regions of the chain.
- By comparing the various acetate-requiring strains concerning the components they lack and the partial reactions they can perform the sequence of many of the components in the chain has been elucidated.
When it has become confirmed that genes have relation with the phenotype of the organism via structural and enzymatic proteins, the synthesis of which depends on them (genes), then it becomes necessary for us to have some basic knowledge about the structure of proteins.
Structure Of Proteins
Proteins, like nucleic acids, are polymers and they consist of a sequence of conjoined monomeric α-amino acids (AA) units of the general type H2N—CH(R)—COOH.
- In biological systems about 40 different amino acids are usually found, but most biological proteins contain only 20 kinds of amino acids.
- These twenty kinds of naturally occurring amino acids have been classified into the following four categories by Lchninger
(1970)—Amino acids with non-polar R groups—Alanine (ala), Valine (Val), Leucine (leu), Isoleucine (file), Proline (pro), Phenylalanine (phe), Tryptophan (Trp), Methionine (met); Amino acids with uncharged polar R groups—Glycine (gly), Serine (ser), Threonine (thr), Cysteine (Cys), Tyrosine (Tyr), Asparagine (asn), Glutamine (Glu); Amino acids with charged polar groups at piI 6.0 to 7.0—
- Basic amino acids (positively charged at piI 6.0)—Lysine (lys), Arginine (arg), Histidine (his);
- Acidic amino acids (negatively charged at pi 1 6.0)—Aspartic acid (asp), Glutamic acid (Glu).
Here R stands for radical.
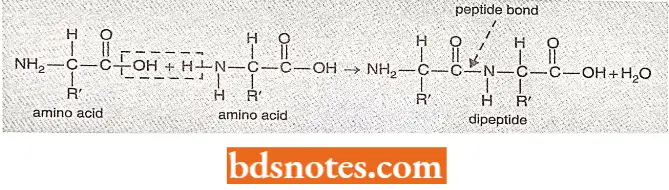
- Peptide linkages and polypeptides, α-amino acids are capable of forming polypeptide chains through the association of the amino (—NH2) group of one amino acid and the hydroxyl (—OH) group of the carboxyl (COOH) radical of another amino acid.
- The resultant amide (—CONH) bonds or linkages between amino acids of a polypeptide chain are called peptide bonds or linkages.
- The respective number of peptide bonds in a peptide or amino acidic chain determines the name of that peptide as, a dipeptide has two such bonds, a tripeptide has three such peptide bonds and a polypeptide has more than two such peptide bonds.
- A polypeptide chain may have a variable number of amino acids according to the specific protein of a species. Further, protein molecules may have different numbers of polypeptide chains.
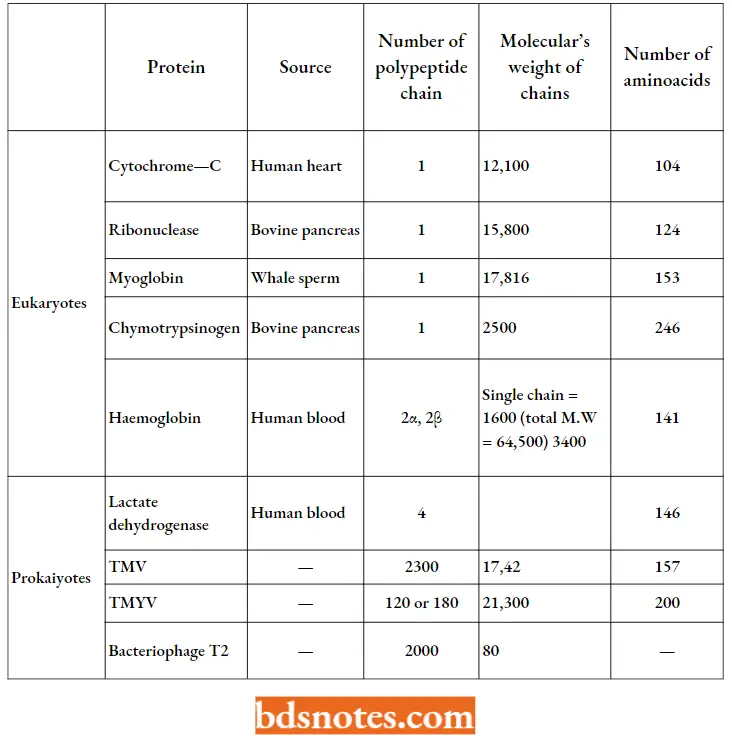
Composition And Size Of Some Important Proteins:
- Each polypeptide chain can be considered as starting from an amino acid with a free —NH2 group (N-terminal) and ending with one free carboxyl (COOH) group (C-terminal).
- In the formation, of polypeptide chains, each amino acid contributes an identical peptide group to the backbone of the molecule (—NH. CH.CO—) together with a distinguishing (R) group.
- Each kind of protein, thus, has a unique number and sequence of such R groups which determine the functional possibilities of the protein molecule.
Structural Levels Of Proteins
Biochemists have recognized the following structural levels of proteins:
Primary structure. The linear sequence of amino acid components in a polypeptide chain constitutes the primary molecular structure of a protein. The newly synthesized protein molecule has this structure.
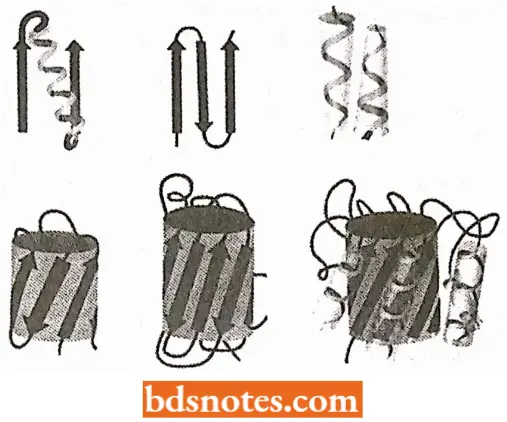
Secondary structure. The functional groups of the polypeptide chain tend to form H-bonds between them. Thus, when the sections of the primary polypeptide chain are twisted or coiled into a helix, hydrogen bonds are formed between the —CO group and an —OH group of amino acids facing each other due to the coiling of a polypeptide chain, and thus, a secondary structure of protein results.
- In biological proteins, the polypeptide chain remains coiled either in alpha Iα)like shape or in beta (β) like shape and consequently are called α-helix and β-helix respectively.

Tertiary structures. To compress the very long spiral chain in a globular form, there occur extensive folding over and bending of the helices, which results in tertiary structural features.
- The tertiary configuration is stabilized by certain intra-chain bonds, especially disulfide (—S— S—) bridges between cysteine residues of a polypeptide chain.
- When two cysteine amino acids confront each other, they are linked 3. by a disulfide bond to form a double amino acid chain.
- For example, insulin enzyme contains two polypeptide chains held together by two — S—S— bridges.
- Ribonuclease enzyme, on the other hand, contains four intra-chain disulfide bridges when giving a three-dimensional arrangement of the molecule.
- Because, tertiary structure permits interaction over a vast surface and creates interstices between the polypeptide chains into which particular reaction patterns can fit with optimal accuracy, most tertiary proteins act like catalysts. Enzymes have tertiary structures.
Quaternary structure. Sometimes, equivalent inter-chain bonds or bridges link together two or more otherwise independent polypeptide chains and so join subunits into a complex quaternary or multimeric edifice. Most proteins with a molecular weight higher than, 20,000 possess such a quaternary structure and are composed of more than one polypeptide structure.
- The best example of a quaternary molecular structure is the hemoglobin (Hb) molecule which has two a -helix and two (β-helix, each with a molecular weight of about 16,000 and each associated with one haem group.
- The four chains are disposed in tetrahedral arrangement but barely touch one another, being held together mainly by H-bonds and by electrostatic attractions.
- The molecular weight, number of total amino acids, and number of polypeptide chains of some biologically important proteins have been tabulated.
Species Specificity of Proteins: Each cell contains hundreds of different proteins and each kind of cell contains the same proteins that are unique to it.
- There is evidence that every species of plant, animal, and microorganism has certain proteins that differ from those of every other species.
- The degree of difference in the proteins of two species depends upon the evolutionary relationship of the form involved.
- Organisms less closely related by evolution have proteins that differ more markedly than those of closely related forms.
- These facts formulate the theory of species specificity which states that the constituent proteins are characteristic of each species differing at least slightly from those of more distantly related species.
One Gene-One Polypeptide Chain Hypothesis
The hypothesis of one gene-one protein-one phenotype relationship has not been found correct by later findings of physiological genetics.
- There is various evidence in human beings and in other organisms that suggests that the synthesis and structure of a single protein molecule can be controlled by two or more genes.
- Recent works have confirmed that a single gene controls the production of a single polypeptide chain of protein molecules, therefore, the concept of one gene-one enzyme, now is termed as the concept of one-gene one-polypeptide and it is exemplified by the following examples:
Human Haemoglobin Genetics
The respiratory pigment, hemoglobin (Hb) of human red blood cells, chemically is a conjugated protein that is composed of four separate polypeptide chains and four iron-containing ring compounds (heme groups).
- In general, the globin protein of hemoglobin may be composed of four different kinds of polypeptide chains called alpha (α), beta (β), gamma (γ), and delta (δ. Each kind of polypeptide chain differs from each other in number and arrangement of amino acids.
- 98 percent of hemoglobin of normal adult people (HbA) consists of two α-polypeptide chains (each of 141 A A) and two β-chains (each of 146 AA) so that the molecule can be written as αAαA βAβA (574AA, M. Wt. 67,000). HbA is thus a protein polypeptide and is therefore called a heteropolymeric protein.

- The rest 2 percent of hemoglobin in normal adult human blood contains two delta (δ) polypeptide chains instead of beta chains, such as haemoglobin (HbA2) can be symbolized αAαAδAδA.
- The hemoglobin of fetal blood has two gammas (γ) polypeptide chains instead of β-polypeptide chains, such hemoglobin (HbF) can be symbolized as αAαAγFγF.
- From these variants of hemoglobin [viz., αAαA βAβA (HbA), αAαAδAδA (HbA2), and αAαAγFγF (HbF) it becomes evident that aA polypeptide chains are controlled by a single gene, while, β, γ, δ polypeptide chains are controlled by different forms (alleles) of another single gene or some closely linked genes.
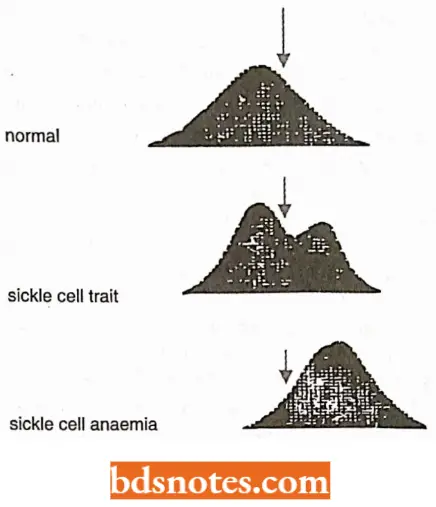
- In 1949 Pauling and his co-workers reported that the formation of an abnormal hemoglobin (hemoglobin S, HbS) which differed in its electrophoretic mobility from normal hemoglobin (HbA) was the cause of hereditary disease sickle-cell anemia in humans.
- The red blood cells of individuals suffering from this fatal form of hemolytic anemia undergo a reversible alteration in shape when the oxygen tension of the plasma falls slightly, and they assume elongated, filamentous, and sickle-like forms.
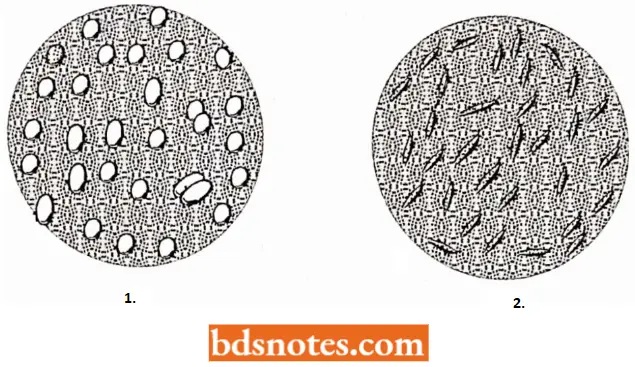
- Such rod cells show u greatly shortened life span since they tend to dump together and alien causing vascular obstruction and are rapidly destroyed. Individuals with sickle-cell anemia are homozygous for an abnormal gene (HS).
- The blood cells of normal individuals (of genotypes HA/HA) never sickle, but the blood cells of perfectly healthy heterozygotes (HA/Hs) can be caused to sickle provided the oxygen concentration is drastically reduced.
- Shortly after the discovery of hemoglobin S(HbS), the study of certain families that showed an aberrant system of inheritance of sickle-cell anemia led to the identification of a second abnormal hemoglobin C (HbC), HbC also tends towards sickle-cell anemia.
- The HbS and HbC traits were later on found to be controlled by two alleles of a single gene.
- The heme moieties of the different hemoglobins are identical, and it was suggested, therefore, that the differences in the net electric charge of HbA, HbS, and Hbc might result from modification in the amino acid sequence.
- Ingram (1957) showed subsequently that the difference between hemoglobins A, S, and C (i.e., HbA, HbS, and HbC) resulted from amino acid substitutions. Each β-chain of hemoglobin has 146 positions, each occupied by a specific amino acid.
Position six is occupied by a negatively charged glutamic acid in HbA; whereas neutral valine and positively charged lysine are substituted at this position, in the case of HbS and HbC, respectively, as illustrated in the following manner:
- HbA: val—his—leu—thr—pro—glu—glu—lys…..
- HbS: val —his—leu—thr—pro—val—glu—lys…
- HbC: val—his—leu—thr—pro—lys—glu—lys…..
These amino acid substitutions result in Hbs and Hbc molecules bearing a net charge relative to HbA of 2+ and 4+.
- Such charge differences account for the observed differences in the electrophoretic points of the three molecules.
- The available information, therefore, indicates that in the case of hemoglobin, at least two chromosomal genes are concerned with the formation of a single protein and that at each locus there occurs a variety of alleles.
- The genetics of hemoglobin also gives evidence in support of the gene’s relation with the molecular structure of proteins (i.e., arrangement of amino acids in the polypeptide chains of a protein).
- Sickle cell disease is caused by replacing a polar glutamic acid residue with a nonpolar valine on the outer surface of the β-globin molecule.
- This causes increased intermolecular adhesion, leading to aggregation of deoxyhemoglobin and distortion of the red cell.
- Sickled red blood cells have decreased survival time (leading to anemia) and tend to occlude capillaries, leading to ischemia and infraction of organs downstream of the blockage (Strachan and Reid,1996).
- An example of Sickle cell anemia shows the drastic influence of a change in a single amino acid in a protein having 560 amino acids (2 chains of a and 2 chains of 3, each 140 amino acids).
- There may however be other cases, where changes in one or two amino acids do not affect the phenotype as in insulin.
Phenotypic Expression Questions and Answers
Questions 1. A single gene change blocks the normal conversion of phenylalanine to tyrosine
- Is the mutant gene expected to be pleiotropic?
- Explain.
Answer:
- Yes.
- A block would result in the accumulation of phenylalanine and a decrease in the amount of tyrosine, which would be expected to result in several phenotypic expressions.
Questions 2. How can normal hemoglobin (hemoglobin A) and hemoglobin S be distinguished?
Answer:
These hemoglobins can be distinguished by the mobility of molecules in an electric field (electrophoretic mobility) and by determining the amino acid sequences of their Polypeptides.
Questions 3. Why is sickle-cell anemia called a molecular disease?
Answer:
- The label ‘molecular disease’ became common in speaking of sickle-cell anemia because its molecular basis (the substitution of a valine residue at amino acid position number 6 in the β-chain) was recognized quite early during the emergence of the science of molecular biology.
- Most if not all inherited diseases probably have very similar molecular bases. We just don’t know what the molecular defects are in the most instances.
Questions 4. Assuming that the beta hemoglobin chain? what mechanisms might explain the differences that now exist in these two chains? What changes in DNA and mRNA codons would account for the differences that have resulted in, unlike amino acids in corresponding positions?
Answer: Mutations: transition, transversions, and frameshifts.
Phenotypic Expression Multiple Choice Questions And Answers
Questions 1. In which of the following disorders, blood has a defective hemoglobin?
- Hemophilia
- Hematuria
- Hematoma
- Sickle cell anemia
Answer: 4. Sickle cell anemia
Questions 2. Sickle cell anemia is caused by to substitution of
- Valine at 6th position in P chain of Hb by valine
- Glutamic acid at the 6th position in the β chain of Hb by valine
- Glutamic acid at 6th position in a chain of Hb
- Valine at 6th position in α chain of Hb by glutamic acid
Answer: 2. Glutamic acid at the 6th position in the β chain of Hb by valine
Questions 3. The first protein to have its primary structure determined was
- Urease
- Insulin
- Glucagon
- Histone
Answer: 2. Insulin
Questions 4. The enormous diversity of protein molecules is mainly due to the diversity of
- Peptide bonds
- Amino groups on the amino acids
- R group on the amino acids
- Amino acid sequences within the protein molecule
Answer: 4. Amino acid sequences within the protein molecule
Questions 5. Albinism is a congenital human disorder resulting from the lack of the enzyme
- Catalase
- Fructokinase
- Tyrosinase
- Xanthine oxidase
Answer: 3. Tyrosinase
Questions 6. Inability to convert dihydroxy phenylalanine into melanin lead, to which of the conditions given below
- Alkaptonuria
- Albinism
- Cretinism
- Glycosuria
Answer: 2. Albinism
Questions 7. Who proposed the central dogma of molecular biology?
- Beadle and Tatum
- Watson and Crick
- Klug
- Crick
Answer: 4. Crick
Questions 8. One of these can not be prepared directly from DNA
- Another DNA
- mRNA
- Protein
- tRNA
Answer: 3. Protein
Questions 9. Which one of the following is not a fibrillar protein ?
- Elastin
- Collagen
- Myosin
- Albumin
Answer: 4. Albumin
Questions 10. The ribozyme is a RNA
- Without sugar
- With extra phosphate
- With enzymatic activity
- Without phosphate
Answrer: 3. With enzymatic activity

Leave a Reply