Fluorides
Question 1. Write short note on shoe leather survey.
Or
Write short answer on shoe leather survey.
Answer.
- In the history of fluoride, in 1931 the element fluoride was identified as the ‘mysterious factor’ responsible for mottled enamel. Fluoride was established as the positive factor for mottling of enamel by “Trendy H Dean” by a survey known as “shoe leather survey”.
- Shoe leather survey is the study of relationship between fluoride concentration in drinking water, mottled enamel and dental caries.
- In 1931 US Public Health Service appointed Dr Trendly H Dean to continue the work of McKay in determining the extent and severity of mottled enamel.
- Dean conducted survey among 22 cities of 10 states of USA on a total population sample of 5,824 children and give following report of mottling of enamel at various concentration of fluoride:
- 4 PPM or more: Presence of signs of discrete pitting
- 3 PPM or more: Mottling was widespread
- 2 to 3PPM: Teeth show dull chalky appearance
- 1 PPM or less: No mottling of any aesthetic significance.
- He also reported that incidence of dental caries in these teeth was less as compared to non-fluoridated teeth.
- A higher concentration of fluoride in water is directly related to the severity of enamel mottling.
- With the excitement of early results, fluoridation of water is started in USA.
- It was then believed that addition of 0.1 PPM fluoride to the drinking water result in maximal reduction in permanent tooth caries experience of 6 to 8 year old children.
- With similar conditions, a significant but not a complete reduction in permanent tooth dental caries experience had been achieved in 12 to 14 year aged individuals.
Read And Learn More: Public Health Dentistry Question And Answers
Question 2. Describe the mechanism of action of fluoride in the prevention of caries. Also describe in detail the professionally applied topical fluoride.
Or
Define topical fluoride. Explain in detail professionally applied topical fluoride.
Or
Write short note on role of topical fluoride in prevention of dental caries.
Or
Write short note on importance of topical application of fluoride.
Or
Write short note on importance of fluoride in dentistry.
Or
Describe the role of systemic and topical fluorides in the prevention of dental caries.
Or
Write short note on fluoride in preventive dentistry.
Answer. Topical fluoride is used to describe those delivery systems which provide fluoride for local chemical reaction to exposed surfaces of erupted dentition.
Following is the role of topical fluoride in prevention of dental caries:
Fluoride inhibits demineralization
- Fluoride incorporated developmentally into the normal tooth mineral is insufficient to have a measurable effect on acid solubility.
- If fluoride is present in the plaque fluid at the time that the bacteria generate acid it will travel with the acid down into the subsurface of the tooth, adsorb to the crystal surface and protect it against being dissolved.
Enhances remineralization
- Saliva is “supersaturated” with calcium and phosphate providing a driving force for mineral to go back into the tooth.
- Partially dissolved crystals act as “nucleators” for remineralization.
- Fluoride acts to speed up this remineralization process by adsorbing to the surface and acting to bring calcium and phosphate ions together, and is preferentially included in the chemical reaction that takes place, producing a lower solubility end-product inhibits plaque bacteria.
Interference with microorganisms
- In high concentrations, fluoride is bactericidal and helps in reduction of plaque.
- In lower concentrations fluoride is bacteriostatic and helps controlling the growth of bacteria without destroying them. Fluoride lodges in plaque and inhibit bacterial enzymes responsible for acid metabolism.
Increased rate of posteruptive maturation
Fluoride increases the rate of mineralization of hypomineralized areas. Newly erupted teeth have hypomineralized areas which are prone to dental caries. Fluoride increases rate of mineralization and prevent from caries.
Modification in tooth morphology
Direct relationship exists between amount of fluoride ingested during tooth development and incidence of dental caries. If fluoride is ingested during tooth development the diameter and cusp depth of teeth are smaller. These changes decrease the caries susceptibility of teeth by making them more self cleansing.
Lowers free surface energy
Fluoride lead to the substitution of hydroxyl ions and reduces free surface energy and so indirectly reduces the deposition of pellicle and the subsequent plaque formation.
Desorption of protein and bacteria
Hydroxyapatite crystals consist of both positive and negative receptor sites. Acidic protein group bind to calcium site and basic to phosphate site. Fluoride inhibits binding of acidic protein to hydroxyapatite, and displays its beneficial effects.
Suppress the flora
Fluoride inhibits bacterial metabolism by oxidizing the thiol group present inside the bacteria.
Professionally Applied Topical Fluoride
Professionally applied fluoride products are those medicaments which are typically dispensed by dental professional in the dental office and involve use of high fluoride concentration product ranging from 5000 to 19000 PPM.
Classification Of Topical Fluoride
- Aqueous solutions.
- Sodium fluoride 2%
- Stannous fluoride 8%.
- Fluoride gels.
- Acidulated phosphate fluoride – 1.23%.
- Fluoride varnishes.
- Duraphat
- Fluorprotector
- Carex.
- Fluoride prophylactic paste.
- Foam.
- Restorative materials containing fluoride.
- Fluoride containing devices (slow release).
Fluoride Varnishes
A fluoride varnish is a professionally applied adherent material.
Mechanism of Action
When varnish is painted on the tooth surface, it acts as a fluoride depot from which fluoride ions are continuously released. These ions react with hydroxyapatite over a longer period of time as varnish is not quickly washed away by saliva. This leads to deeper penetration and significant anticaries effect.
Types of Fluoride Varnishes
- Duraphat
- It was the first fluoride varnish to be tested.
- It is a viscous resinous lacquer which is to be applied on dry, clean tooth.
- It hardens into yellowish brown coating in presence of saliva.
- Fluorprotector
- It is a clear polyurethane based product containing 7000 ppm fluoride from organic compound.
- It leaves a clear transparent film on tooth.
- It has a range of efficacy between 1% and 17%.
- Carex
- It has a lower fluoride concentration.
- It has same efficacy as duraphat has.
Method of Varnish Application
- Oral prophylaxis is done.
- Teeth are dried and but not isolated with cotton rolls as varnish sticks to cotton.
- First lower arch is taken up for application and then upper arch as saliva collects rapidly on the lower arch.
- Dispense a small amount of varnish (0.3 mL–0.5 mL, or 2 drops, for the entire primary dentition) to the applicator dish or pad.
- Application is done with single tufted brush starting with proximal surfaces (Dental floss can be used to ensure that the varnish reaches interproximal areas).
- Since varnish sets rapidly when they come in contact with saliva, no drying is necessary.
- After application, patient is made to sit with mouth open for 4 minutes.
- Patient is instructed not to rinse or drink anything for 1 hour, and not to eat anything solid and avoid brushing till next morning. Patient is advised to take liquids or semisolids only, as contact between varnish and tooth surface is maintained for about 13 hours. It is for prolonged interaction between fluoride and enamel.
Foam
- Foam based agents reduces the risk of fluoride overdosage.
- Mainly APF used in foams.
- It is lighter than the conventional gel and should be used in small amounts.
Fluoride Prophylaxis Paste
- Surface enamel consists of high levels of fluoride as compared to inner layers. So the prophylaxis removes the fluoride rich layer.
- Prophylaxis pastes having fluoride replenish the lost fluoride and there is small but significant net gain in concentration of fluoride.
Restorative Materials Containing Fluoride
- Fluorides releasing dental restorative material are also available, that provide site specific protection.
- In general, the rate of fluoride release from such materials is not constant but exhibits a relatively rapid initial rate, which decreases with time.
- These materials may feature greater longevity, a reduced incidence of marginal failure, an elevated concentration of fluoride in contingent plaque, together with an antibacterial action when compared with nonfluoride releasing material.
- The purpose of adding fluoride to restorative material is to capture its anticariogenic property.
- The fluoride ions are slowly released from the materials.
- Fluoride may be released from dental restorative materials as part of the setting reaction, or it may be added to the formulation with the specific intention of fluoride release.
- Fluoride containing restorative materials includes glass ionomer cements, resin modified glass ionomer cements. Polyacid modified resin composites (compomers), resin composites, fissure sealants and dental amalgam.
- Fluoride releasing components have included fluoro aluminosilicate glasses (FAG), stannous fluoride (SnF2), organic amine fluorides (OAF) and ytterbium fluoride (YbF).
Fluoride Containing Devices
This a topical system of slow and constant fluoride release. They help in prevention of dental caries.
These fluoride containing devices are of three types viz:
- Copolymer membrane type
- Glass bead
- Mixture of sodium fluoride and hydroxyapatite.
Question 3. Write short note on Knutson’s technique of fluoride application.
Or
Write short note on Knutson’s technique of topical fluoride application.
Or
Write short note on Knutson’s technique.
Answer. By using Knutson’s technique neutral sodium fluoride or 2% sodium fluoride application is done.
Recommended Ages
It is recommended that a series of 4 weekly applications of 2 percent NaF be given at ages 3,7,11 and 13, coinciding with the eruption of different groups of primary and permanent teeth.
Method of Application
- Cleaning and polishing of teeth is done.
- Teeth are isolated with cotton rolls and dried with compressed air.
- Teeth can be selected quadrant wise.
- 2% aqueous NaF solution is applied with cotton applicator for 3 minutes.
- Procedure is repeated for remaining quadrants until all of the teeth are treated.
- Second, third and fourth applications are recommended at intervals of approximately 1 week and they are preceded by cleaning and polishing.
- Patient is advised to avoid rinsing, drinking and eating for next half hour.
Question 4. Write note on Muhler’s solution.
Answer. Muhler’s solution is available in powder form either in bulk containers or preweighed capsules.
The recommended and approved concentration is 8%.
Method of Preparation
- The solution has to be freshly prepared as they are not stable.
- It can be prepared by dissolving 0.8 g of powder in 10 mL of distilled water.
- The solution is acidic, with a pH of about 2.4–2.8.
- The left over solution should be discarded after application.
Method of Application
- Cleaning and polishing of teeth is done.
- Teeth are isolated with cotton rolls and dried with compressed air.
- Freshly prepared Stannous fluoride solution is applied using cotton applicator. Care should be taken that all teeth surfaces are treated.
- Repeated loading of cotton applicator should be done and swabbing is continuously done so as to keep tooth surface moist for 4 minutes.
- Patient is allowed to expectorate after cotton rolls are removed.
Advantage
Recommended frequency is 6–12 months interval much less as compared to NaF.
Disadvantages
- Solution should be prepared fresh each time before use.
- Solution produces bitter metallic taste.
- It may lead to gingival irritation.
- It causes brown pigmentation of teeth in hypocalcified areas and around margins of restoration.
Question 5. Write in brief on APF gel.
Or
Write short note on acidulated phosphate fluoride.
Answer. It is also called as Brudevold solution or acidulated phosphate fluoride
- APF was introduced by Brudevold and his co-workers at Forsyth Dental Center.
- APF developed on the basis of the concept that slightly demineralized enamel acquires more fluoride than unaffected enamel.
- APF developed by acidulated with orthophosphoric acid and buffered to a pH of 3.
Method of Preparation
To prepare a gel, a geling agent methyl cellulose is to be added to the solution and the pH is adjusted between 4 and 5.
An aqueous solution of APF is prepared by dissolving 20 g of sodium fluoride in 1 liter of 0.1 M phosphoric acid and to this is added 50% hydrofluoric acid to adjust the pH at 3.0 and fluoride ion concentration at 1.23%.
Technique of Application-Brudevold Technique
- Do a thorough prophylaxis and dry the teeth.
- Fill the upper and lower trays with APF gel.
- Insert the upper and lower trays simultaneously into the patient mouth and have the patient bite down tightly for 4 min.
- Thixotropic gel displays a high viscosity at lower shear rates and a very low viscosity at higher shear rates.
- The clinical importance of this is that the gel thins outunder biting forces and more easily penetrates between the teeth.
- Conversely, when it is not under stress it remains in the tray and does not tend to run down the patient throat.
- Instruct the patient not to eat, drink or rinse for 30 min.
Acidulated Phosphace Fluoride Advantages
- Require only two applications in a year and is thus suited for most dental office routine.
- The gel preparation can be self-applied and thus, the cost of application also gets reduced.
- It is stable and need not to be freshly prepared each time.
- It has capability of depositing fluoride in enamel deeply as compared to sodium fluoride and stannous fluoride.
Acidulated Phosphace Fluoride Disadvantages
- Practical difficulties like the teeth should be kept wet for 4 minutes.
- It is acidic and sour and bitter in taste.
- It cannot be stored in glass containers because it may remove mineral from each glass.
- Repeated or prolonged exposure of porcelain or composite restorations of APF can result in the loss of materials, surface roughening and possible cosmetic changes.
Question 6. Write short note on topical fluoride.
Or
Write short answer on topical fluoride.
Or
Write in brief on topical fluoridation.
Answer. Topical fluorides.
- Topical fluorides are applied directly on teeth.
- Topical fluoride causes interaction of fluoride with minerals in teeth.
- Topical fluorides are the delivery systems which provide fluoride to local chemical reaction at exposed surfaces of erupted teeth.
- Topical fluoride act by inhibiting demineralization and promoting remineralization.
- It is divided into two categories, i.e. professionally applied and self applied.
Self Applied Topical Fluoride
- It consists of fluoride dentifrices, gels and rinses used by an individual.
- Depending on their way of usage the preparations consists of 0.5–3.4 mg of fluoride.
Fluoride Gels
- Self applied fluoride gel consists of neutral sodium fluoride and APF with fluoride concentration of 5000 ppm and stannous fluoride with concentration of 1000 ppm.
- These gels are applied once a day.
- Patient should brush their teeth for one minute with the gel.
- He should not swallow the excess gel.
- Patient should have to rinse with water after completion of brushing.
- It is not recommended in children below 6 years.
Fluoride Mouthrinses
- Sodium fluoride mouthrinses are commonly used.
- For home use they are prepared by dissolving 200 mg sodium fluoride tablet in 5 teaspoons of fresh clean water.
- Patient should use rinse and expectorate method.
Question 7. Write short note on fluoride containing dentifrices.
Answer.
Dentifrices
- Dentifrices are aids for cleaning and polishing tooth surface.
- The paste consist of a solid abrasive, suspended in a liquid phase, containing detergent, humectants, binding agents, flavor, color, preservative and a fluoride source.
Functions of Fluoridated Dentifrices
Dentifrices have been classified as cosmetic or therapeutic, based on whether they contain a drug for the treatment or prevention of a dental disease.
Physiomechanical Function
It may reduce the cariogenicity of the remaining plaque and prevent the formation of thicker, more cariogenic plaque by rinsing or flushing action.
Chemical Function
The chemical functions of fluoride dentifrices are based on the potential anticaries mechanism of fluoride.
Fluoride Compounds in Dentifrices
- Sodium fluoride dentifrices:
-
- Because of the success of the topical application ofsodium fluoride in preventing caries formation, dentifrices containing this agents were formulated.
- The food and drug administration (1973) approved a sodium fluoride dentifrice formulated with calcium pyrophosphate.
- Stannous fluoride dentifrices:
- Stannous fluoride dentifrices became the first dentifrices recognized by Food and Drug administration as an effective tooth decay prevention product in 1955.
- But, it becomes less popular because it causes stainingof teeth, pigmentation of hypoplastic areas and margins of restoration.
- Its low pH and high concentration of stannous ion causes a metallic, astringent taste, which is difficult to accept mostly by children.
- Monofluorophosphate:
- In 1981, it became the most widely used agent for formulation of caries preventive dentifrices.
- It did not cause staining of teeth.
Mechanism of Action
- Some study suggests that the monofluorophosphate anion has anticariogenic properties due to exchange of its phosphate group in the apatite crystals.
- Some studies suggest that the caries inhibiting action of monofluorophosphate is due to release of fluoride ion in oral environment.
- Amine fluoride dentifrices: The dentifrices have markedly superior properties concerning enamel solution rate reduction, fluoride uptake by enamel and antiglycolytic activity in plaque.
Question 8. Write short note on systemic fluoride.
Answer. Systemic fluoride consists of low concentration of fluoride to teeth and is given for long period of time.
- Systemic fluoride circulates via bloodstream and is supplied to developing teeth.
- Various types of systemic fluorides are:
- Community water fluoridation
- Salt fluoridation
- Milk fluoridation
- Fluoride tablets/drops/lozenges
Milk Fluoridation
- Milk fluoridation was introduced by Zeigler in Swiss city of Winterthur in 1953.
- Milk fluoridation consists of addition of measured quantity of fluoride to bottled or packed milk which was to be taken by children.
- For fluoridation of milk sodium fluoride is added to the milk in form of concentrated aqueous solution in a fixed volume.
- If the requirement of fluoride is 1 mg per day, concentration of fluoride in milk is set up at 5ppm.
- Fluoridated milk should be distributed to children inkindergarten and nursery schools.
Fluorides Tablets/Drops/Lozenges
- Fluorides tablets/drops/lozenges should be prescribed to individual patients or in school or in home-based prevention.
- They are prescribed by the dentist or pediatrician.
- Sodium fluoride is the most common agent used.
- Fluoride drops should be given to infants.
- Fluoride tablets and lozenges should be chewed, swallowed and swished.
Question 9. Write in brief water fluoridation.
Or
Discuss water fluoridation.
Answer. Water fluoridation is defined as “controlled adjustment of the concentration of fluoride in a communal water supply so as to achieve maximum caries reduction and a clinically insignificant level of fluorosis”.
- Optimum level of fluoride in water is approximately 1 ppm.
- In water fluoridation, fluoride is ingested in public water supplies.
- Water fluoridation is of two types, i.e. community water fluoridation and school water fluoridation.
Community Water Fluoridation
- This is the controlled adjustment of fluoride in communal water supply so as to achieve maximum caries reduction and a clinically insignificant level of fluorosis.
- Water fluoridation may be defined as an upward adjustment of the concentration of fluoride ion in public water supply.
- The concentration of fluoride ion in the water may be consistently maintained at 1 ppm by weight.
Fluoridation Advantages
- Large number of population is benefited.
- Water consumption is regular.
- Fluoridated drinking water provides more resistance to enamel which prevent dental decay.
- It is very economical.
Fluoridation Disadvantages
- It interferes with the human rights.
- Any other mode is not considered.
- Common source of water supply is not present.
School Water Fluoridation
- By this the fluoride is available to children in optimal amount.
- Amount of the fluoride which is added in the drinking water is more as compared to community water supply.
- Currently recommended level of fluoride is 4.5–6.3 ppm of fluoride in school water supply.
- The concentration of fluoride in water is 4.5 times more, because the children remain in school for short time and also consume less water.
- School water fluoridation provides topical effects on erupting teeth.
- It prevents dental caries in school children.
Advantages
- Recipient need not to put any effort.
- There is reduction in dental caries.
- Very minimum equipment is needed.
- It is very economical.
Disadvantages
- If children are not going to school, they should not receive the benefit.
- All children are not visiting the school.
- Amount of drunken water should not be regulated.
- Continuous careful monitoring is needed.
Question 10. Write short note on salt fluoridation.
Answer. Salt fluoridation was introduced by Wespi in year 1948 in Switzerland.
- It is the method of preventing dental caries on mass level.
- Salt fluoridation consists of measured addition of fluoride at the time of manufacture of salt for community consumption.
- Level of fluoride in salt should be 200, 250 and 350 mg per kg of salt.
Production of Fluoridated Salt
Production of fluoridated salt takes place by two processes, i.e. batch processing and continuous processing.
Batch Processing
- In this there is addition of fixed amount of fluoride in fixed amount of refined salt.
- Sodium fluoride is used for the batch processing.
- In batch processing most homogeneous distribution of fluoride in a ton of salt is obtained after mixing it for 20 minutes.
Continuous Processing
- This procedure occurs in large plants.
- In this a dose of concentrated fluoride solution is sprayed via nozzle on the salt which is passing on a conveyer belt.
- In this method potassium fluoride is commonly used.
Advantages
- Community water supply is not needed.
- Individuals can accept or reject it.
- Nonfluoridated salt as like noniodide salt can be available to individuals who are in need.
Disadvantages
- High salt intake can lead to hypertension.
- Require high technical expertise.
- Only refined salt is required.
- Difficulty occur if multiple drinking sources are available.
Question 11. What is fluoridation? Why fluoridation is needed in water, toothpaste and restorative materials? Give criteria for the fluorosis index.
Answer. Fluoridation is the controlled adjustment of a fluoride compound to a public water supply in order to bring the fluoride concentration up to a level which effectively prevent caries.
Need of Fluoridation in Water
- This is the controlled adjustment of fluoride in communal water supply so as to achieve maximum caries reduction and a clinically insignificant level of fluorosis.
- The concentration of fluoride ion in the water may be consistently maintained at 1 ppm by weight.
- Fluoridation helps in prevention of dental and root caries.
- Provide life-long benefits and decrease dental cost.
- Since the concentration of fluoride in drinking water is adjusted and continuously monitored, adverse effects are avoided.
- It is the easiest method to prevent dental caries or disease and reach more people at less cost.
Need of Fluoridation in Toothpaste
- It may reduce the cariogenicity of the remaining plaque and prevent the formation of thicker, more cariogenic plaque by rinsing or flushing action.
- Chemical functions of fluoride toothpaste are based on the potential anticaries mechanism of fluoride.
Need of Fluoridation in Restorative Materials
- Fluorides releasing dental restorative material are also available, that provide site specific protection.
- In general, the rate of fluoride release from such materials is not constant but exhibits a relatively rapid initial rate, which decreases with time.
- These materials may feature greater longevity, a reduced incidence of marginal failure, an elevated concentration of fluoride in contingent plaque, together with an antibacterial action when compared with non-fluoride releasing material.
- Purpose of adding fluoride to restorative material is to capture its anticariogenic property.
- Fluoride ions are slowly released from the materials.
- Fluoride may be released from dental restorative materials as part of the setting reaction or it may be added to the formulation with the specific intention of fluoride release.
Question 12. Write short note on acute fluoride toxicity.
Or
Write short answer on fluoride toxicity.
Answer. It results due to rapid ingestion of excessive fluoride.
- Most frequent adverse effect of topical fluoride is nausea.
- Other symptoms are abdominal cramps, vomiting, diarrhea, increased salivation, dehydration and thirst.
- If first aid is not given to patient he/she may die.
- In acute fluoride toxicity death is due to the blocking of normal cellular metabolism.
- Death occurs due to cardiac failure or respiratory paralysis.
- Toxic dose is 16-32 mg/kg body weight.
- Certainly lethal dose is 32–64 mg of fluoride mg/kg body \weight.
Sign and Symptoms
- Nausea, vomiting, abdominal pain, diarrhea
- Excessive salivation and mucosal discharge
- Generalized weakness and carpopedal spasm
- Weak thread pulse and hypotension
- Depression of respiratory centre
- Cardiac arrhythmia
- Coma and death
Management
- If patient had taken fluoride less than 5 mg/kg.
- Give calcium orally i.e. milk is given to relieve gastrointestinal symptoms. Observe for few hours.
- But induction of vomiting is not necessary.
- If patient had taken fluoride more than 5 mg/kg but less than 15 mg/kg body weight
- Gastric emptying should be done by induction of vomiting with emetics. If patient have depressed gag reflex induction of vomiting should be contraindicated. Endotracheal intubation is performed before gastric lavage.
- Orally calcium should be given.
- Admit patient to hospital and observe for few hours.
- If patient had taken fluoride more than 15 mg/kg
- Admit patient to the hospital immediately.
- Induce the vomiting.
- Cardiac monitoring should be started.
- 10% calcium gluconate solution should be slowly administer intravenously.
- Levels of calcium and potassium should be monitored.
- Adequate urine output should be maintained with help of diuretics.
- Supportive measures should be taken for shock.
Chronic Fluoride Toxicity
- It occurs from long term ingestion of small amount of fluoride.
- Effect of the chronic fluoride toxicity on enamel is dental fluorosis and other problems such as skeletal fluorosis also occur.
Question 13. Write in brief on defluoridation.
Or
Define defluoridation. Describe different methods of defluoridation.
Or
Write short note on defluoridation.
Or
Write short answer on defluoridation.
Answer. Defluoridation is the process of removing excess naturally occurring fluoride from drinking water in order to reduce the prevalence and severity of dental fluorosis.
Characteristics of Defluoridation
- It should be cost-effective.
- It is easy to handle/operate by rural population-the major sufferer.
- Independent of input fluoride concentration, alkalinity, pH, temperature.
- It should not affect taste of water.
- It should not add other undesirable substances (e.g.aluminum) to treated water.
Methods of Defluoridation
Methods for defluoridation are:
- Based upon ion exchange process or adsorption method.
- Based upon addition of chemicals to water during treatment or additive method.
- Other methods.
Adsorption Method
- This is carried out by running fluoride over contact beds where the fluoride is removed by ion exchange or chemical reactions with the agents comprising the bed matrix. Bed regeneration is essential.
- The most commonly used adsorbents are activated alumina and activated carbon.
- The fluoride removing efficiency of activated alumina gets affected by hardness and surface loading (the ratio of total fluoride concentration to activated alumina dosage). Chloride does not affect the defluoridation capacity of activated alumina.
- The process is pH specific, so pH of the solution should be between 5.0 and 6.0 because at pH >7 silicate and hydroxide becomes stronger competitor of the fluoride ions for exchange sites on activated alumina and at pH less than 5, activated alumina gets dissolved in acidic environment leading to loss of adsorbing media. The process is highly selective, but it has low adsorption capacity, poor physical integrity, requires acidification and pretreatment and its effectiveness for fluoride removal reduces after each regeneration.
- Mckee and Johnston in 1934, investigated the use of powdered activated carbon for fluoride removal and achieved good results. The process is pH dependent with good results only at pH 3.0 or less. Therefore, the use of this material is expensive due to need of pH adjustment.
Ion-exchange Method
Fluoride can be removed from water supplies with a strongly basic anion exchange resin containing quarternary ammonium functional groups. The removal takes place according to the following reaction:
\(\text { Matrix }-\mathrm{NR}_2+\mathrm{Cl}^{-}+\mathrm{F}^{-} \rightarrow \text { Matrix }-\mathrm{NR}_2+\mathrm{F}^{-}+\mathrm{Cl}^{-}\)The fluoride ions replace the chloride ions of the resin. This process continues until all the sites on the resin are occupied. The resin is then backwashed with water that is supersaturated with dissolved sodium chloride salt. New chloride ions then replace the fluoride ions leading to recharge of the resin and starting the process again. The driving force for the replacement of chloride ions from the resin is the stronger electronegativity of the fluoride ions.
Additive Method
In this chemicals are added to precipitate the fluoride or to absorb fluoride on other precipitated compounds. Chemicals usually used are lime (calcium oxide), magnesium compounds and aluminum sulfate (alum). Precipitation step is followed by mixing, flocculation, settling and filtration. The filtering beds need to be cleaned constantly of the accumulating sludges.
Other Methods
Other methods are:
- Coagulation-precipitation.
- Membrane process.
Coagulation-precipitation
- Lime and alum are the most commonly used coagulants.
- As a first step, precipitation occurs by lime dosing which is followed by a second step in which alum is added to cause coagulation. When alum is added to water, essentially two reactions occur. In the first reaction, alum reacts with some of the alkalinity to produce insoluble aluminium hydroxide [Al(OH)3]. In the second reaction, alum reacts with fluoride ions present in the water. The best fluoride removal is accomplished at pH range of 5.5-7.5.
Membrane Process
- In the recent years, reverse osmosis membrane process has emerged as a preferred alternative to provide safe drinking water without posing the problems associated with other conventional methods.
- Reverse osmosis is a physical process in which the contaminants are removed by applying pressure on the feed water to direct it through a semipermeable membrane.
- The process is the reverse of natural osmosis as a result of the applied pressure to the concentrated side of the membrane, which overcomes the natural osmotic pressure.
- Reverse osmosis membrane rejects ions based on size and electrical charge.
- The factors influencing the membrane selection arecost, recovery, rejection, raw water characteristics and pretreatment.
- Efficiency of the process is governed by different factors such as raw water characteristics, pressure, temperature and regular monitoring and maintenance, etc.
- There are two types of membranes that can remove fluoride from water: Nano filters and reverse osmosis.
- Nanofilter is a relatively low pressure process that removes primarily the larger dissolved solids as compared to reverse osmosis. Conversely, reverse osmosis operates at higher pressures with greater rejection of all dissolved solids.
Question 14. Write short note on Nalgonda technique.
Or
Write in brief on Nalgonda’s technique of water defluoridation.
Answer. Nalgonda technique is a technique of defluoridation of water.
- Nalgonda technique was developed by the National Environmental Engineering Research Institute (NEERI) at Nagpur in 1974.
- The process comprises addition in sequence of sodiumacuminate or lime, bleaching powder and filter alum to the fluoride water followed by flocculation, sedimentation and filtration.
- The technique is extremely useful both for domestic as well as for community water supplies.
Mechanism of Defluoridation by Nalgonda Technique
- Rapid mix: Rapid mixing is an operation uniformly dispersed throughout single or multiple phase system. It occurs within the period of 30–60 seconds with speed of 10–20 rpm.
- Flocculation: Flocculation is the second stage of formation of settable particles from destabilized colloidal sized particle and it is achieved by gentle and prolonged mixing. It occurs within the period of 10 to 15 minutes with speed of 2 to 4 rpm.
- Sedimentation: It is the separation from the water by gravitational setting of suspended particles that are heavier than water.
Factors that influencing the sedimentation are:- Size, shape, density and nature of the particle.
- Viscosity, density and temperature of water.
- Velocity of flow.
- Surface over flow rate
- Effective depth of settling zone
- Filtration: It is a process for separating suspended and colloidal impurities from water by passage through porous media. Flocculated water allowed to settle and filters via earth candles. Treated water consists of 1 ppm or less of fluoride.
Salient Features of Nalgonda Technique
- No regeneration of media.
- No handling of caustic acid and alkalies.
- Simplicity of design, construction, operation and maintenance.
- Little wastage of water and least disposal problem.
- Needs minimum of mechanical and electrical equipment.
- No energy except muscle power for domestic equipment.
- Only the readily available chemicals used in conventional municipal water treatment are required.
- It is adaptable for the domestic usage.
- There is high efficient removal of the fluoride to desired levels.
- It is effective even when the dissolved solids are above 1500 mg/L and hardness above 600 mg/L.
Question 15. What do you mean by defluoridation.
Answer. Defluoridation is the process of removing excess naturally occurring fluoride from drinking water in order to reduce the prevalence and severity of dental fluorosis.
Question 16. Write short note on fluoride varnishes.
A fluoride varnish is a professionally applied adherent material.
Mechanism of Action
When varnish is painted on the tooth surface, it acts as a fluoride depot from which fluoride ions are continuously released. These ions react with hydroxyapatite over a longer period of time as varnish is not quickly washed away by saliva. This leads to deeper penetration and significant anticaries effect.
Types of Fluoride Varnishes
- Duraphat
- It was the first fluoride varnish to be tested.
- It is a viscous resinous lacquer which is to be applied on dry, clean tooth.
- It hardens into yellowish brown coating in presence of saliva.
- Fluorprotector
- It is a clear polyurethane based product containing 7000 ppm fluoride from organic compound.
- It leaves a clear transparent film on tooth.
- It has a range of efficacy between 1% and 17%.
- Carex
- It has a lower fluoride concentration.
- It has same efficacy as duraphat has.
Method of Varnish Application
- Oral prophylaxis is done.
- Teeth are dried and but not isolated with cotton rolls as varnish sticks to cotton.
- First lower arch is taken up for application and then upper arch as saliva collects rapidly on the lower arch.
- Dispense a small amount of varnish (0.3 mL to 0.5 mL, or 2 drops, for the entire primary dentition) to the applicator dish or pad.
- Application is done with single tufted brush starting with proximal surfaces (Dental floss can be used to ensure that the varnish reaches interproximal areas).
- Since varnish sets rapidly when they come in contact with saliva, no drying is necessary.
- After application, patient is made to sit with mouth open for 4 minutes.
- Patient is instructed not to rinse or drink anything for 1 hour, and not to eat anything solid and avoid brushing till next morning. Patient is advised to take liquids or semisolids only, as contact between varnish and tooth surface is maintained for about 13 hours. It is for prolonged interaction between fluoride and enamel.
Question 17. Describe in detail the method of preparation, application, mechanism of action, advantages and disadvantages of Knutson’s solution.
Answer. Knutson’s solution is 2% neutral sodium fluoride.
Method of Preparation
The solution is prepared by dissolving 20 g of sodium fluoride powder in 1 L of distill the water in a plastic bottle.
Mechanism of Action
As Knutson’s solution is applied to the surface of teeth it reacts with hydroxyapatite crystals in enamel to form calcium fluoride. As a thick layer of calcium hydroxide forms it interfere with further dissolution of fluoride from topical fluoride solution to react with hydroxyapatite and block further entry of fluoride ions. This sudden stop of entry of fluoride is known as “chocking off effect”. Fluoride now leaches from calcium fluoride and this calcium fluoride act as a reservoir for release of fluoride. Calcium fluoride reacts with hydroxyapatite crystals and form fluoridated hydroxyapatite. This formed hydroxyapatite increases the concentration of fluoride over the surface of enamel which leads to the tooth surface resistant to caries.
Knutson’s solution Advantages
- Its taste is good and acceptable to all.
- It is stable in plastic bottle and no need to prepare fresh solution every time.
- It is nonirritating to oral tissues.
- It does not lead to tooth discoloration.
- Once the solution is applied to teeth it should be left for 3 minutes, so that it become dry and in this way the dentist in public health programme can undergo multiple chair procedure.
Knutson’s solution Disadvantages
Patient should have to visit four times to the dentist in very short period of time.
Question 18. Write short note on community water fluoridation.
Answer. Most common form of systemic fluoride administration is addition of fluoride to public water supply.
- Optimal level of fluoride in water for protection against dental caries is approximately 1 ppm.
- Water fluoridation is defined as “controlled adjustment of the concentration of fluoride in a communal water supply so as to achieve maximum caries reduction and a clinically insignificant level of fluorosis”.
- Dean and various other scientists conducted studies which showed that fluoride was associated with lower prevalence of dental caries and there was a sound basis for hypothesis that introduction of fluoride by water supply result in lower communal prevalence of dental caries.
- Fluoride compounds used in the water fluoridation are fluorspar, sodium fluoride, silicofluofides, sodium silicofluoride, hydrofluosilicic acid.
- For community water fluoridation the commonly used methods are a volumetric dry feeder system, acid feed system, saturator feed system and Venturi fluoridation system. Out of all these, acid feed system is most commonly used.
Water Fluoridation Advantages
- Large number of population is benefited.
- Water consumption is regular.
- Fluoridated drinking water provides more resistance to enamel which prevent dental decay.
- It is very economical.
Limitation of Community Water Fluoridation
- For community water fluoridation there is a need of well established central piped water distribution system. But in most of the developing countries it is not present. In rural areas they are rarely seen.
- For community water fluoridation there is need of support of top health authorities and government.
- Any other mode is not considered.
- It interferes with human rights.
- Common source of water supply is not present.
Question 19. Classify topical fluorides used in dentistry. Write in detail about the preparation, concentration, method of application and age groups for application of topical sodium fluoride.
Answer.
Classification of topical fluorides used in dentistry:
Professionally applied topical fluoride
- Aqueous solutions
- Sodium fluoride—2%
- Stannous fluoride—8%.
- Fluoride gels
- Acidulated phosphate fluoride—1.23%.
- Fluoride varnishes
- Duraphat
- Fluor protector
- Carex.
- Fluoride prophylactic paste.
- Foam.
- Restorative materials containing fluoride.
- Fluoride containing devices (Slow release).
Self-applied topical fluorides
- Fluoride dentifrices
- Fluoride mouthrinses
Concentration of Topical Sodium Fluoride
Concentration of topical sodium fluoride is 2% which provides reduction in dental caries about 30%.
Preparation of Topical Sodium Fluoride
2% topical sodium fluoride is prepared by dissolving20 g of sodium fluoride powder in 1 L of distilled water in the plastic bottle. Since the solution has basic pH, so it remains stable in plastic bottle. It should not be stored in glass bottle as fluoride ion of prepared solution reacts with silica of glass and form silicon fluoride which reduces availability of free active fluoride.
Age Group for Application of Topical Sodium Fluoride
It is recommended that a series of four weekly applications of 2% sodium fluoride is given at ages of 3, 7, 11 and 13 years, coinciding with the eruption of different groups of primary and permanent teeth.
Method of Application of Topical Sodium Fluoride
- Cleaning and polishing of teeth is done.
- Teeth are isolated with cotton rolls and dried with compressed air.
- Teeth can be selected quadrant wise.
- 2 % aqueous NaF solution is applied with cotton applicator for 3 minutes.
- Procedure is repeated for remaining quadrants until all of the teeth are treated.
- Second, third and fourth applications are recommended at intervals of approximately 1 week and they are preceded by cleaning and polishing.
- Patient is advised to avoid rinsing, drinking and eating for next half an hour.
Question 20. Write short answer on controlled water fluoridation studies.
Answer. Following are the controlled water fluoridation studies:
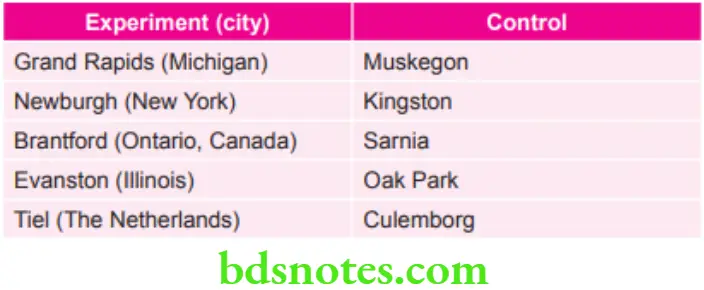
Grand Rapids–Muskegon Study
- This study was carried out on 25th January 1975.
- In this, sodium fluoride was added to the supply of Grand Rapids water and Muskegon was the control.
- Effects of 61/2 years of fluoridation in grand Rapids were reported by Arnold et al in 1953.
- Results depict that caries experience of 6 year old Grand Rapid child was almost half to that of six year old Muskegon child.
Newburgh–Kingston Study
- This study was carried out on 2nd May 1945.
- In this sodium fluoride was added to drinking water of Newburg situated on the Hudson river. Kingston town was taken as control.
- After ten years of fluoridation, it was reported that the DMF rate had decreased from 23.5% to 13.9%, this confirming the caries inhibitory property of fluoride in the drinking water.
The Brantford–Sarnia–Stratford fluoridation Caries Study
- This study was carried out in Canada.
- A project was undertaken in Brantford, Ontario, where fluoride was added to the water supply in June 1945. Community of Sarnia was taken as control town. In addition, the community of Stratford, where level of fluoride in natural drinking water is 1.3 ppm was used as an auxiliary control.
- After 17 years of fluoridation in Brantford, it was observed that caries experience was similar to that which occurs in the natural fluoride area of Stratford and was estimated as 55% lower than in the control town of Sarnia.
Evanston–Oak Park Study
- Fluoridation experiment was began in Evanston, Illinois and also in the nearby community of Oak Park is taken as the control town.
- After 14 years of fluoridation in Evanston, it was observed that there was a decrease of 49% in the DMF values.
- Evanston-Oak Park study was presented as the most detailed data of all the fluoridation studies.
Tiel–Culemborg Fluoridation study
- Drinking water in Tiel was fluoridated to a level of 1.1 ppm and Culemborg with water fluoride level of 0.1 ppm was taken as control.
- After 13 years of fluoridation, the number of anatomical sites of teeth affected by dental caries was 58% lower in Tiel as compared to Culemborg.
Question 21. Write short answer on fluor protector.
Answer.
- Fluor protector is a dental varnish.
- Fluor protector was developed in 1970.
- It is a clear polyurethane based product which consists of 7000 ppm fluoride from an organic compound difluorosilane.
- Fluor protector has polyurethane lacquer which is dissolved in chloroform and difluorosilane at the concentration of 2% by weight which is equal to 0.32% fluoride inside the liquid.
- It should be dispensed in 1 mL of ampule. Each ampule has 6.21 mg of fluoride.
- A steep concentration gradient from surface to interior is observed for both silicon and fluoride. This can imply an association between uptake of fluoride and silicon and provide the suggestion that silanes are an effective medium of transport of fluoride in the enamel.
- Fluor protector leaves a clear transparent film of teeth.
- Fluor protector has range of efficacy between 1% and 17% but its clinical effectiveness is always questionable.

Leave a Reply