Fluids, Electrolytes And Nutrition
A good understanding of the physiology of fluids and electrolytes is fundamental to the practice of surgery. Most surgical conditions are associated with changes in this balance and it is only appropriate that these are identified and treated effectively.
Read And Learn More: Clinical Medicine And Surgery Notes
Normal Physiology
The human body consists of about 50–70% liquids and 30–50% solids by weight. The liquid portion varies with age, sex and body habitus. The variation is the result of individual differences in the fat content of the body which contains very little water.
- Hence, thin individuals have greater total body water (TBW) content as compared to obese individuals. Similarly, the TBW is about 50% in women and 60% in men. Neonates have up to 80% TBW.
- Of this total body water, intracellular water constitutes 40% of body weight (2/3 of TBW) and the extracellular portion, 20% of body weight (1/3 of TBW). The interstitial fluid and plasma portions of extracellular fluid constitute 15% and 5% of body weight respectively.
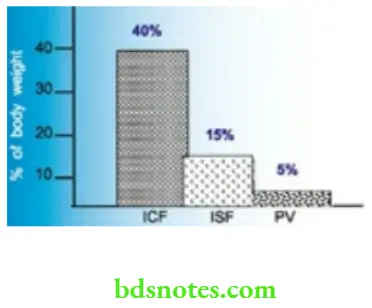
Composition of Body Fluids
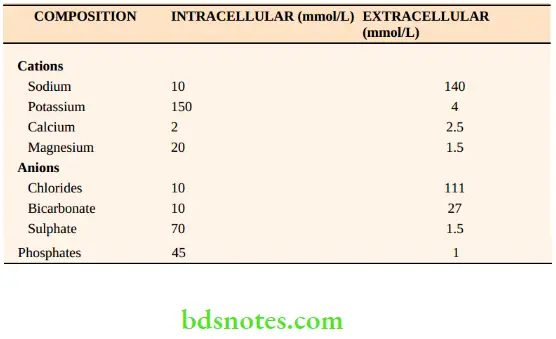
These fluid compartments are separated by semipermeable membranes allowing their fluid composition to be maintained within distinct limits. shows the composition of the intracellular and extracellular fluid compartments. The composition of the intracellular compartments may vary according to the tissue, for example. fat contains very little water.
The tonicity of plasma is determined by the solutes, sodium and its corresponding anions, chlorides and bicarbonate, together with substances such as glucose, urea and proteins. These particles are osmotically active and hence, tonicity is described in terms of Osmolality (mOsm/kg HO2).
Osmolarity is the concentration of a solution in terms of osmoles (or osmoles) of solute per litre of solution (solute + water). Osmolality is the concentration of a solution in terms of osmoles (or osmoles) per kilogram of solvent. The osmolality is independent of the temperature of the solution and the volume of the solute. Hence, osmolality is the preferred term in clinical practice.
The osmolality of a solution can be measured in two ways:
1. By using the depression of the freezing point of the solution: A solution of 1 osm/kg freezes at −1.86° C. Normal plasma freezes at −0.54° C
\(\begin{aligned} \text { Plasma osmolality } & =\frac{0.54}{1.86} \times 10^3 \mathrm{mOsm} / \mathrm{kg} \mathrm{H}_2 \mathrm{O} \\ & =290 \mathrm{mosm} / \mathrm{kg} \mathrm{H}_2 \mathrm{O} \end{aligned}\)2. By estimating the solute concentration: Osmolality can be estimated from the concentration of major solutes of plasma
\(\begin{array}{r} \text { Osmolality }=2 \times[\mathrm{Na}]+\frac{[\text { Glucose }(\mathrm{mg} \%)]}{18} \\ \frac{+[\text { Blood urea }(\mathrm{mg} \%)]}{6} \end{array}\)Example: If a patient’s sodium concentration is 140 mmol/L, blood glucose concentration is 180 mg% and blood urea is 30 mg%, his plasma osmolality can be calculated as follows.
\(\begin{aligned} \text { Osmolality } & =280+10+5=295 \mathrm{mOsm} / \mathrm{kg}\mathrm{H}_2 \mathrm{O} \\ & =2 \times[\mathrm{Na}]+\frac{180}{18}+\frac{30}{6} \end{aligned}\)From the equation, it is evident that sodium contributes the most to the osmolality of plasma.
A change in osmolality is usually due to changes in sodium. The normal range of plasma osmolality is 285–300 mOsm/kg HO2.
Plasma Colloidal Osmotic Pressure
The plasma proteins normally do not pass out of the capillaries into the interstitium. These raise the plasma osmotic pressure above that of the interstitial fluid by an amount referred to as colloidal osmotic pressure (plasma oncotic pressure). The normal plasma colloidal osmotic pressure is 25 mmHg. Albumin is responsible for 75% of this oncotic pressure.
The body has mechanisms to regulate and maintain the volume of fluids, their concentration and composition within narrow limits to maintain homeostasis. Hence, a systematic assessment of the fluid status of a patient involves the assessment of body fluid volume, its concentration and its composition in that order.
Water Regulation (Regulation of volume)
The primary methods of body water regulation are:
- Regulating the volume of liquid ingested: When the extracellular fluid volume reduces, the thirst centre in the hypothalamus is stimulated which encourages the person to ingest more water.
- Regulating the volume of urine excreted: This is regulated by plasma antidiuretic hormone (ADH). A reduction in plasma volume releases ADH from the posterior pituitary which in turn acts on the ADH receptors in the collecting tubules of the kidney, resulting in increased reabsorption of water and reduced production of urine. ADH release may also be stimulated by plasma osmolality and angiotensin.
Disturbances Of Volume
A decrease in the circulating volume is called hypovolaemia and an increase, hypervolaemia.
Hypovolaemia
This is common in surgical patients. The assessment of acute loss of blood volume is detailed in Chapter 7. The reduction in blood volume due to loss of water can be in the following ways:
- Gut—vomiting, diarrhoea, fistulae
- Skin and lungs—0.5 ml/kg/h normally, increases by 12% for every °C rise in temperature
- Sequestration of fluid in third space
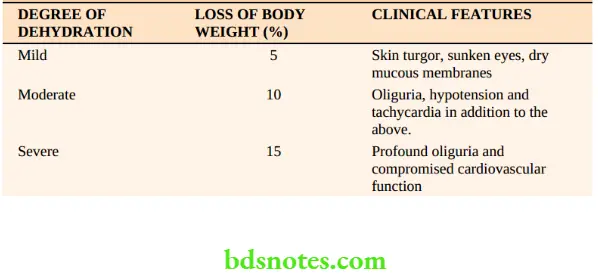
Assessment Of Dehydration
This is a clinical assessment based upon
- History: Severity and duration of loss of fluid
- Examination: Thirst, dryness of the mucosa, loss of skin turgor, orthostatic hypotension, tachycardia, reduced jugular venous pressures and decreased urine output in the presence of normal renal function. Dehydration can be classified as follows.
Laboratory Assessment
Haemoconcentration leads to falsely elevated haemoglobin, packed cell volume estimations and increased blood urea concentration. The kidneys reabsorb more water than usual leading to increased urine osmolality (> 650 mOsm/kg).
Hypervolaemia
Hypervolaemia Causes
- Excessive infusion of intravenous fluids
- Retention of water in abnormal conditions such as cardiac, renal and hepatic failure
- Absorption of water as during transurethral resection of prostate using distilled water.
Hypervolaemia Diagnosis
- History and physical examination can lead to the cause.
- Physical examination—Distended neck veins, pedal oedema, body weight gain
- Circulatory overload:
- Hypertension, tachycardia, pulmonary oedema
- Confusion, restlessness, convulsions and coma
The development of these signs depends on the rate and volume of fluid overload, renal function and cardiovascular reserve.
Hypervolaemia Management
- Treat the cause
- Restriction of water and salt
- Diuretics (or dialysis, if necessary) to remove excess water
Regulation Of Sodium Concentration
Water constitutes the major component of all body fluids but the composition varies with the fluid compartment. The most abundant cation of extracellular fluid is sodium and is the prime determinant of ECF volume. 90% of the ECF osmolality is due to sodium. The human body has no known mechanism to regulate sodium intake.
The body regulates sodium output by:
- Regulating glomerular filtration rate
- Regulating plasma aldosterone levels
The addition or loss of water produces a change in the concentration of the solute. The quantity of solute relative to the volume of water is thereby increased (ECF is concentrated) or decreased (ECF is diluted) with the loss or addition of water respectively.
Changes in concentration are generally changes in water balance rather than changes in sodium regulation. Since the changes in volume and concentration are interdependent and the changes in water content are not easily measured, an estimate of the fluid volume and concentration is usually made using the measured sodium levels and serum osmolality.
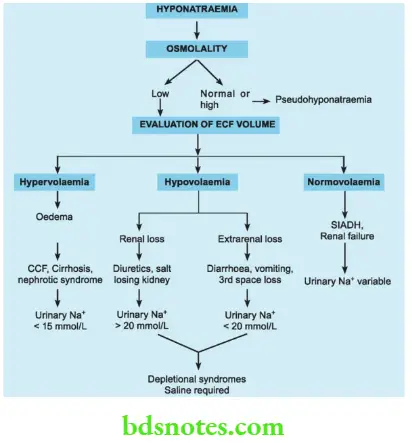
Disturbances In Concentration Hyponatraemia
Hyponatraemia is defined as a sodium level less than 135 mmol/L. It may occur as a result of water retention, sodium loss or both. True hyponatraemia is always associated with low plasma osmolality. It may be associated with expanded, contracted or normal extracellular volume.
Hypervolaemia Causes
Assessment of hypovolaemia should begin with an estimation of the extracellular fluid volume (clinically and if necessary, using central venous catheters). Thus, true hyponatraemia can be of three types: hypervolemic hyponatraemia, hypovolaemic hyponatraemia and normovolaemic hyponatraemia.
Hyponatraemia
Hypervolaemic hyponatraemia may be associated with clinical features of hypervolaemia such as oedema. Acute hypervolaemia, (for example. TURF syndrome —acute absorption of hypotonic fluids into the intravascular compartment) may result in cerebral oedema and pulmonary oedema.
- As plasma osmolality decreases, water moves from the extracellular space into the cells leading to oedema. The expansion of brain cells is responsible for the symptomatology of water intoxication: nausea, vomiting, lethargy, confusion, restlessness etc. If severe ([Na+] < 100 mmol/L), it can result in seizures and coma.
- Chronic hypervolaemia as in congestive cardiac failure, cirrhosis and nephrotic syndrome may manifest with pedal oedema and elevated jugular venous pressures till decompensation occurs. The urinary sodium concentration is less than 15 mmol/L.
Hypervolaemia Treatment
Acute hyponatraemia (duration < 72 h) can be safely corrected more quickly than chronic hyponatraemia. The following factors must be evaluated: patient’s volume status, duration and magnitude of the hyponatraemia and the degree and severity of clinical symptoms.
- Fluid restriction, diuretics, and correction of the underlying condition may be adequate in most cases. A combination of intravenous normal saline and diuresis with a loop diuretic, for example. frusemide also elevates the serum sodium concentration.
- Acute symptomatic hyponatraemia is a medical emergency. It should be treated with hypertonic saline (1.6% or 3%). Concomitant use of loop diuretics increases free water excretion and also decreases the risks of fluid overload.
- The sodium concentration must be corrected to relieve symptoms and to a concentration of 125 mmol/L. With patients who are acutely symptomatic, the treatment goal is to increase the serum sodium by approximately 1–2 mEq/ L/h until the neurologic symptoms subside.
- The correction should be slow and over a period of 12–24 hours with frequent checks of sodium concentration (every 2–4 h) to avoid overcorrection.
- Avoid an absolute increase of serum sodium of more than 15–20 mEq/L in a 24-hour period. If sodium correction is undertaken too rapidly, the resulting osmolality changes in the extracellular fluid can cause central pontine myelinolysis. This condition is serious and can be irreversible.
The following equation can aid in the estimation of a sodium deficit to help determine the rate of saline infusion:
- A litre of normal saline contains 154 mEq sodium chloride (NaCl) and 3% saline 500 mEq NaCl. In chronic severe hyponatremia (i.e. serum sodium <115 mEq/L), the rate of correction should be slow and should not exceed 0.5–1 mEq/L/h, with a total increase not to exceed 10 mEq/L/day.
Hyponatraemia
Hypovolaemia with inappropriate correction with hypotonic fluids such as 5% dextrose may result in hyponatraemia. Hypovolaemia may be due to renal causes such as diuresis or a salt-losing kidney. The urinary concentration of sodium is more than 20 mmol/L in these patients.
Extrarenal loss of volume as in diarrhoea, vomiting or 3rd space loss may result in a urinary concentration of less than 20 mmol/L. All these are termed depletional syndromes and require saline infusion.
Hyponatraemia Treatment
Based upon the volume status, administer isotonic saline to patients with hypotonic hyponatremia who are hypovolaemic to replace the contracted intravascular volume.
Normovolaemic Hyponatraemia
Occasionally, hyponatraemia may exist with normovolemia. In such situations, the plasma osmolality must be estimated. If it is low, renal failure or the syndrome of inappropriate ADH secretion (SIADH) may be considered.
Normovolaemic Hyponatraemia Treatment
- For patients who have hypotonic hyponatraemia and are normovolaemic (euvolaemic), asymptomatic, and mildly hyponatraemia, water restriction (1 L/day) is generally the treatment of choice.
- For instance, a fluid restriction of 1 L/day is enough to raise the serum sodium in most patients. This approach is recommended for patients with asymptomatic SIADH.
- Pharmacological agents can be used in some cases of more refractory SIADH, allowing more liberal fluid intake.
- Demeclocycline is the drug of choice to increase the diluting capacity of the kidneys by achieving vasopressin antagonism and a functional diabetes insipidus.
Pseudohyponatraemia
Occasionally, hyponatraemia is only an apparent one due to the accumulation of other solutes such as glucose, urea, plasma proteins or lipids. The plasma osmolality is either high or normal in these patients. Such hyponatraemia is called pseudohyponatraemia.
- Serum osmolality is governed by contributions from all molecules in the body that cannot easily move between the intracellular and extracellular space. Sodium is the most abundant electrolyte but glucose, urea, plasma proteins and lipids are also important. Normally their concentrations are small and contribute to the plasma osmolality only to a small extent.
- However, when the concentrations of these molecules increase to very high levels, the relative concentration of sodium in a unit volume of serum may reduce. The actual amount of sodium is normal in these patients hence the term pseudohyponatraemia.
- High blood sugar levels or uraemia leads to higher plasma osmolality but high plasma protein or lipid levels are associated with normal plasma osmolality.
Pseudohyponatraemia Treatment
The treatment of hypertonic and pseudo hyponatremia primarily is supportive in the absence of symptoms.
Hypernatraemia
Hypernatraemia is defined as a plasma sodium concentration of more than 150 mmol/L and may result from pure water loss, hypotonic fluid loss or salt gain.
Causes of Hypernatraemia
- Pure water depletion
- Extrarenal loss intake: Failure of water (coma, elderly, postoperative patients) Mucocutaneous loss -Fever
- Renal loss: Diabetes insipidus Chronic renal failure
- Hypotonic fluid loss
- Extrarenal loss: Gastrointestinal (vomiting, diarrhoea) Excessive sweating
- Renal loss :Osmotic diuresis (glucose, urea, mannitol)
- Salt gain: Iatrogenic (sodium bicarbonate, hypertonic saline) Salt ingestion Steroid excess
The hypertonicity of plasma leads to cellular dehydration. Clinical evidence of dehydration may not be apparent until 10–15% of body weight has been lost. Rehydration should be slow to prevent cerebral oedema.
The diagnosis can be established by measuring plasma and urine osmolalities and urine output.
- Uosm > Posm and ↓ urine output → Extrarenal causes (for example diarrhoea, fistulae etc.)
- Uosm > Posm and ↑ urine output→ Osmotic diuresis
- Uosm < Posm and urine output → ↓ ADH or ↓ renal response to ADH.
Hypernatraemia Treatment
- Administration of water orally/nasogastric tube
- Administration of IV fluid −5% dextrose or 0.45% saline
- Change in serum sodium not more than 0.05 to 1 mmol/L/h. Rapid rehydration can cause cerebral oedema.
Disturbances In Composition Of Body Fluids
Potassium Balance
The normal potassium level is 3.5–5.5 mmol/L. Hypokalaemia and hyperkalemia are the two important disturbances.
Hypokalaemia
This is defined as a plasma concentration of potassium less than 3.5 mmol/L.
Hypokalaemia Causes
- Reduced intake
- Tissue redistribution: Insulin therapy, alkalaemia, β adrenergic agonists, familial periodic paralysis.
- Increased loss: Gastrointestinal losses —diarrhoea, vomiting, fistulae
- Renal causes: Diuretics, renal artery stenosis, diuretic phase of renal failure.
Hypokalaemia Symptoms
- Anorexia, nausea
- Muscle weakness, paralytic ileus
- Altered cardiac conduction: Delayed repolarisation, reduced height of ‘T’ wave, presence of ‘U’ wave, wide QRS complexes and arrhythmias.
Hypokalaemia Management
- Diagnosis and treatment of the cause
- Repletion of body stores
- Potassium supplements, in the form of milk, fruit juice, and tender coconut water.
- Syrup potassium chloride orally—15 ml contains 20 mmol of potassium
- If the patient cannot take it orally or the hypokalaemia is severe, intravenous potassium chloride can be given at a rate not exceeding 0.5 mmol/kg/h under electrocardiographic monitoring. A maximum of 200 mmol/ day should not be exceeded in a 70 kg individual.
Hyperkalaemia
This is defined as a plasma concentration of potassium more than 5 mmol/L.
Causes
Hyperkalaemia Clinical features
Vague muscle weakness, flaccid paralysis.
Electrocardiographic changes
- Tall, peaked ‘T’ waves with shortened QT interval (6–7 mmol/L)
- Wide QRS complex, widening and then loss of ‘P’ wave (8–10 mmol/L)
- Wide QRS complex, merge into ‘T’ waves (sine wave pattern)
- Ventricular fibrillation (K+ > 10 mmol/L).
Treatment of hyperkalaemia
- Calcium gluconate (10%) → 10–30 ml.
- Sodium bicarbonate → 1–2 mmol/kg over 10–15 minutes.
- 100 ml of 50 % dextrose with 10–12 units of insulin over 15–20 minutes.
- Hyperventilation
- Salbutamol nebulisation
- Calcium exchange resins
- Peritoneal or haemodialysis.
Hyperkalaemia Causes
- Pseudohyperkalaemia
- In vitro
- haemolysis
- Thrombocytosis
- Tourniquet
- Exercise
- Impaired excretion
- Renal failure
- Hyperaldosteronism
- K+ sparing
- diuretics
- Tissue redistribution
- Tissue damage
- (burns, trauma)
- Acidosis
- Rhabdomyolysis
- Tumour necrosis
- Suxamethonium
- Familial
- Hyperkalaemic
- Periodic paralysis
- Massive
- Intravascular
- Haemolysis
- Excessive input
- Stored blood
- Transfusion
- Excessive
- Intravenous
- Potassium
- Supplementation
Magnesium
- Magnesium is the second most abundant intracellular ion. The normal intracellular concentration of magnesium is about 26 mmol/L and extracellular concentration is 1.5–2.5 mmol/L. Almost 60% of the magnesium is deposited in the skeleton. Magnesium is required as a cofactor for several important enzymatic reactions, including the phosphorylation of glucose within cells and the use of ATP by contracting muscle fibres.
- A daily dietary intake of 0.3–0.4 g (approximately 20–30 mEq) is required. The proximal convoluted tubule reabsorbs magnesium very effectively.
Calcium
- Calcium is the most abundant mineral in the body. 99 per cent is deposited in the skeleton. In addition, calcium ions are important for the control of muscular and neural activities, in blood clotting, as cofactors for enzymatic reactions, and as second messengers.
- Calcium homeostasis reflects a balance between reserves in the bone, rate of absorption across the digestive tract, and rate of loss at the kidneys. The hormones parathyroid hormone (PTH), vitamin D and calcitonin maintain calcium homeostasis in the ECF. Parathyroid hormone and vitamin D raise Ca++ concentrations and calcitonin lowers it.
- Calcium absorption at the digestive tract and reabsorption along the distal convoluted tubule is stimulated by PTH from the parathyroid glands and calcitriol from the kidneys. The average daily requirement of calcium in an adult is 0.8–1.2 g/day.
- Normal serum Ca++ concentration is 9 to 11 mg/dl.
Hypercalcaemia
Hypercalcemia exists when the Ca2+ concentration of the ECF is above 11 mg/dl
Causes Of Hypercalcaemia
- Hyperparathyroidism
- Malignancies of the breast, lung, kidney, or bone marrow
Features
Severe hypercalcaemia (12–13 mg/dl) causes such symptoms as fatigue, confusion, cardiac
arrhythmias, and calcification of the kidneys and soft tissues throughout the body.
Hypocalcaemia
Hypocalcemia exists when the calcium level is less than 9 mg/dl. However, the serum calcium level should be related to the albumin levels. Half the serum calcium is bound to albumin and as albumin levels become low, this bound fraction is lower leading to a low total serum calcium concentration. Free calcium ionic concentration is important for the electrical activity of the nerves and muscles and is the ideal parameter to measure but is not easily available.
Causes Of Hypocalcaemia
- Hypoparathyroidism
- Vitamin D deficiency
- Chronic renal failure
Features
Muscle spasms, sometimes including generalised convulsions, myocardial depression, cardiac arrhythmias, and osteoporosis.
Perioperative Fluid Therapy Normal Maintenance Needs
The average daily requirement of water for an average-sized adult is 2000 ml. In general, a volume of 35–40 ml/kg/day meets the daily maintenance needs.
Patients awaiting anaesthesia and surgery need to be kept fasting for a few hours prior to and after the surgery. Except in very minor surgery, the fluid lost during this period needs to be replaced.
The replacement is as follows
- Fluid requirement during starvation −2 ml/kg/h of starvation. This volume is calculated for hours of fasting and then replaced over 2–3 hours.
- Maintenance requirement −2 ml/kg/h of surgery
- Third space losses:
- 4 ml/kg/h for surgery with minimal dissection, for example, herniorrhaphy
- 6 ml/kg/h for surgery associated with moderate dissection, for example. gastrojejunostomy and vagotomy
- 8 ml/kg/h for surgery associated with a large amount of dissection, for example, Whipple’s procedure.
- Blood loss is replaced by compatible blood transfusion (homologous or autologous) if the haematocrit falls below 25%. Blood loss is replaced with an equal amount of colloids or three times the volume with crystalloids if the haematocrit is > 25% in an otherwise healthy individual. Crystalloids are electrolyte solutions and distribute themselves throughout the body water hence, a larger volume needs to be given.
A patient undergoing surgery should receive fluid deficit due to starvation + maintenance fluids + third space losses + replacement of blood loss (as detailed above). The adequacy of fluid replacement should be checked with haemodynamic stability and urine output in major procedures.
When very large fluid shifts are expected (oncologic surgeries), and the patient has compromised cardiac status or has renal insufficiency, it may be necessary to monitor fluid status using central venous pressure monitoring.
What To Give?
The starvation losses are usually replaced by an infusion of 5% dextrose or 5% dextrose in isotonic saline. The maintenance and third space losses are replaced by the Ringer lactate solution.
Types Of Intravenous Fluids
These can be broadly divided into three groups —crystalloids, colloids and special-purpose solutions.
Crystalloids are essentially solutions of electrolytes in water, for example. Ringer lactate. Some also
contain dextrose, for example. dextrose saline, 5% dextrose, Isolyte P, Isolyte M. They vary in the content of different electrolytes.
Colloids are solutions of large molecules which tend to remain in the intravascular compartment, e.g. gelatine, hetastarch, pentastarch, dextran 40, and dextran 70. They are all plasma expanders since the molecules tend to exert osmotic forces and draw fluid from the interstitial compartment into the intravascular space.
- The colloids vary in their magnitude of volume expansion and duration of action. Dextran 40 reduces the viscosity of blood and maintains blood rheology better.
- Hence, it is used as a continuous infusion after microvascular surgery. It can also interfere with coagulation which along with reduced viscosity helps in maintaining blood supply to free flaps and vascular grafts.
Special Purpose Solutions
Sodium Bicarbonate: It is available as 7.5% (0.9 mEq/ml) and 8.4% (1 mEq/ ml) of sodium bicarbonate. It is used as an alkalinising agent (in metabolic acidosis, hyperkalaemia and forced alkaline diuresis).
The disadvantages of indiscriminate sodium bicarbonate therapy are:
- Increased sodium load
- Alkalosis with a shift of oxygen dissociation curve to the left (increased affinity of haemoglobin to O2, reducing its unloading)
- Increased intracranial pressure and intraventricular haemorrhages in neonates
- Circulatory overload leading to cardiac failure
- Carbon dioxide load leads to respiratory failure.
Mannitol (10% and 20%)—is an osmotic diuretic. Its main use is to reduce intracranial pressure by producing diuresis. It is also used to reduce intraocular pressure. Mannitol expands intravascular volume initially by drawing fluid from the interstitium. This is followed by diuresis. It should be used with caution in patients with cardiac failure, renal failure, etc.
Hypertonic Saline (1.6,3 and 5%): These solutions are available to treat hyponatraemia. 7.5% hypertonic saline is used in the rapid expansion of intravascular volume after major trauma.
Albumin: 4.5% albumin is used as a plasma expander. 20% albumin can be used to replace lost albumin in severe hypoalbuminaemia in addition to plasma expansion.
Perioperative Fluid Therapy In Patients With Disturbed Fluid Balance
Derangements of fluid therapy can be classified as follows.
- Disturbances of volume
- Disturbances of concentration
- Disturbances of composition
In the evaluation of a patient with a suspected problem in fluid and electrolyte or acid—base balance, careful sequential analysis of the volume, concentration and composition (in that order) followed by appropriate therapy protects the patient from severe, perhaps fatal errors in management.

Leave a Reply