Endodontic Medicaments And Irrigants
Endodontics is the specialty of dentistry that manages the prevention, diagnosis, and treatment of the dental pulp and the periradicular tissues that surround the root of the tooth.
The main objectives of root canal therapy are
- Removal of the pathological pulp.
- Cleaning and shaping of the root canal system.
- Three-dimensional obturation to prevent reinfection.
Read And Learn More: Basic Dental Materials Notes
Irrigation is an essential part of root canal debridement because it allows for cleaning beyond what might be achieved by root canal instrumentation alone. Disinfection of the root canal system is one of the primary aims of root canal treatment. This can be achieved through the use of various antimicrobial agents in the form of irrigants (only used for relatively short periods of time) and medicaments (days or several weeks). The main objective of root canal obturation is to achieve a three dimensional well filled root canal with fluid tight seal. It serves to prevent percolation and microleakage of periapical exudate into the root canal space and create a favorable environment for the healing process.
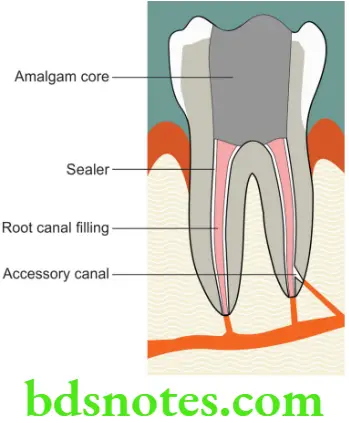
Materials used in endodontics
The materials used in Endodontics may be grouped as follows.
- Root canal irrigants
- Root canal medicaments
- Obturating materials
- Endodontic sealers
Root Canal Irrigants
Several irrigants and irrigant delivery systems are available, all of which behave differently and have relative advantages and disadvantages. Root canal irrigants must not only be effective for dissolution of the organic portions of the dental pulp, but also effectively eliminate bacterial contamination and remove the smear layer (the organic and inorganic layer) that is created on the walls of the root canal during instrumentation.
Root Canal Irrigants Desirable properties
- Irrigants should have a broad antimicrobial spectrum and high efficacy against anaerobic and facultative microorganisms organized in biofilms.
- Dissolve necrotic pulp tissue remnants.
- Inactivate endotoxin.
- Should be nontoxic, noncarcinogenic and nonantigenic.
- Should be able to disinfect and penetrate dentin and its tubules.
- Prevent the formation of a smear layer during instrumentation or dissolve the latter once it has formed.
- Should not have adverse effect on dentin.
- Should not discolor dentin.
- Not irritate or damage vital periapical tissue (caustic or cytotoxic effects).
- Should not weaken tooth structure.
- Should not affect the sealing ability of filling materials.
Root Canal Irrigants Function of irrigants
Irrigants are used to clean the root canal and are used along with the shaping instruments. The functions of irrigants include
- Rinsing and flushing (helps remove debris).
- Lubricant function to reduce instrument friction during preparation.
- Dissolve inorganic tissue (dentin).
- Dissolve organic matter (dentin collagen, pulp tissue, biofilm).
- Penetrate to the peripheries of the root canal system.
- Kill bacteria and yeasts (antimicrobial).
Root Canal Irrigants Classification
Chemically inactive irrigants
- Water
- Saline
- Local anesthetic solution
Chemically active irrigants
- Tissue dissolving agents [e.g. Sodium hypochlorite (NaOCl)]
- Oxidizing agents [e.g. Hydrogen peroxide (H2O2), Sodium hypochlorite]
- Antibacterial agents (e.g. Chlorhexidine, NaOCl, MTAD, Iodine)
- Chelating agents (e.g. EDTA)
Chemically Active Irrigants
Sodium Hypochlorite
- Sodium hypochlorite is used widely as a household bleach and disinfectant. In dentistry, sodium hypochlorite (NaOCl) is a popular as an irrigating solution for Endodontic use.
Sodium Hypochlorite Available as
- Solutions in various concentrations between 0.5% and 6% in bottles.
Sodium Hypochlorite Chemical reaction
NaOCl ionizes in water into Na+ and the hypochlorite ion, OCl–. The OCl– ion in turn combines with water to form Hypochlorous acid (HOCl).
\(\mathrm{NaOCl}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Na}^{+}+\mathrm{Cl}+2 \mathrm{HO}\)In commercial NaOCl solutions, the following species are in equilibrium
\(\mathrm{H}^{+}+\mathrm{OCl}^{-} \leftrightarrow \mathrm{HOCl}\) \(\mathrm{HOCl}+\mathrm{Cl}^{-}+\mathrm{H}^{+} \leftrightarrow \mathrm{Cl}_2+\mathrm{H}_2 \mathrm{O}\)- Hypochlorous acid is responsible for the antibacterial activity; the OCl ion is less effective than the undissolved HOCl. Hypochlorous acid disrupts several vital functions of the microbial cell resulting in cell death. At acidic and neutral pH, chlorine exists predominantly as HOCl, whereas at high pH of 9 and above, OCl predominates.
- It is a potent antimicrobial agent, killing most bacteria instantly on direct contact. It also effectively dissolves pulpal remnants and collagen, the main organic components of dentin. Hypochlorite is the only root canal irrigant of those in general use that dissolves necrotic and vital organic tissue. Although hypochlorite alone does not remove the smear layer, it affects the organic part of the smear layer, making its complete removal possible by subsequent irrigation with EDTA or citric acid (CA). It is used as an unbuffered solution at pH 11 in the various concentrations mentioned earlier, or buffered with bicarbonate buffer (pH 9.0), usually as a 0.5% (Dakin solution) or 1% solution.
- Sodium hypochlorite is the most important irrigating solution and the only one capable of dissolving organic tissue, including biofilm and the organic part of the smear layer. It should be used throughout the instrumentation phase.
Sodium Hypochlorite Biological considerations
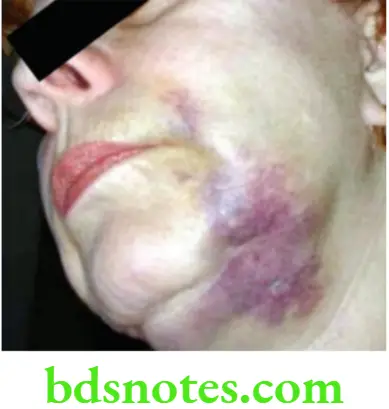
- Extrusion of NaOCl into periapical tissues can cause severe injury to the patient. To minimize NaOCl accidents, the irrigating needle should be placed short of the working length, fit loosely in the canal and the solution must be injected using a gentle flow rate. Constantly moving the needle up and down during irrigation prevents wedging of the needle in the canal and provides better irrigation. The use of irrigation tips with side venting reduces the possibility of forcing solutions into the periapical tissues. Treatment of NaOCl accidents is palliative and consists of observation of the patient as well as prescribing antibiotics and analgesics.
Sodium Hypochlorite Storage
- Sodium hypochlorite is generally not utilized in its most active form in a clinical setting. For proper antimicrobial activity, it must be prepared freshly just before its use. It should be stored at room temperature and not exposed to oxygen for extended periods of time. Exposure of the solution to oxygen and light can inactivate it significantly.
Sodium Hypochlorite Disadvantages
- Unpleasant taste
- Relative toxicity
- Inability to remove smear layer (inorganic)
Chlorhexidine
- Chlorhexidine has a broad-spectrum antimicrobial action and relatively little toxicity. Chlorhexidine (CHX) however, lacks the tissue-dissolving ability. It penetrates the cell wall and attacks the bacterial cytoplasmic or inner membrane or the yeast plasma membrane. It may be used in concentrations between 0.2% and 2%. Its activity is pH dependent and is greatly reduced in the presence of organic matter.
Hydrogen Peroxide
- It is a clear, colorless liquid used in a variety of concentrations. 1%–30% H2O2 is active against viruses, bacteria, and yeasts.


Functions and actions
- It produces hydroxyl free radicals (OH), which attack several cell components such as proteins and DNA. In endodontics, H2O2 has long been used because of its antimicrobial and cleansing properties. It has been particularly popular in cleaning the pulp chamber from blood and tissue remnants, but it has also been used in canal irrigation.
MTAD
- The acronym MTAD stands for mixture of tetracycline isomer, acid and detergent (doxycycline, citric acid, and the detergent Tween-80). It has sustained antibacterial activity. It has a low pH of 2.15. MTAD solubilized dentine well (70%) and pulp tissue to a lesser extent (50%). It is an alternative to EDTA.
EDTA (Ethylenediaminetetraacetic Acid)
- EDTA (17%, disodium salt, pH 7) has little if any antibacterial activity. It effectively removes smear layer by chelating the inorganic component of the dentine. It aids in the mechanical canal shaping.

RC-PREP
- RC-Prep is composed of EDTA and urea peroxide in a base of Carbowax. It is not water soluble. Interaction of the urea peroxide in RC-Prep with sodium hypochlorite, produces a bubbling action thought to loosen and float out dentinal debris.

Intracanal Medicaments
- If root canal treatment cannot be finished in a single visit, root canals are dressed with medicaments. A medicament is an antimicrobial agent that is placed inside the root canal between treatment appointments in an attempt to destroy remaining microorganisms and prevent reinfection.
Functions of intracanal medicaments
Intracanal Medicaments Primary functions
- Antimicrobial activity
- Antisepsis
- Disinfection
Intracanal Medicaments Secondary functions
- Hard-tissue formation
- Pain control
- Exudation control
- Resorption control
Intracanal Medicaments Desirable properties
- Antibacterial
- Penetrate dentinal tubules
- Control exudation or bleeding
- Biocompatible
- Eliminate pain
- Induce calcific barrier
- Have no effect on temporary restoration
- Should be radiopaque
- Should not stain tooth
Types of intracanal medicaments
- Phenol and related volatile compounds
- PBSC paste
- Sulfonamide and sulfathiazole
- Corticosteroid-antibiotic combination
- Calcium hydroxide
- N2
- Halogens
- Quaternary ammonium compounds
Phenol And Related Compounds
- Phenol
- Eugenol
- CMCP
- Cresatin
- Formocresol
- Glutaraldehyde
- Cresol
- Beechwood
- Creosote
- Thymol
Phenolic Compounds
- Phenol is a protoplasm poison and produces necrosis of soft tissue. However, because it has a strong inflammatory potential, at present it is rarely used as an intracanal medicament.
Eugenol
- Eugenol is the chemical essence of the oil of clove. It is both antiseptic and an anodyne. It is a constituent of most root canal sealers and used as a temporary sealing material. It inhibits intradental nerve impulses.
Parachlorophenol (PCP)
- It is a substitution product of phenol. The aqueous solution of PCP penetrates deeper into the dentinal tubules, than camphorated chlorophenol. A 1% solution of PCP is capable of killing many of the microorganisms in the root canal. It may, however, produce mild inflammation.
Formocresol
- This is a combination of formalin and cresol in the ratio of 1:2 or 1:1. Formalin is a strong disinfectant that combines with albumin to form an insoluble, nondecomposable substance and fixes the tissues. It is used as a dressing for pulpotomy to fix the retained pulp tissue. Formocresol is a nonspecific bacterial medicament most effective against aerobic and anaerobic organisms found in root canals. Formocresol is also mutagenic and carcinogenic.
- It is placed on a cotton pellet, squeeze dried and then placed in the pulp chamber of the tooth in treatment. The vapors will penetrate the entire canal preparation.
Cresatin
- Cresatin or metacresylacetate has both antiseptic and obtundent properties. But the antimicrobial effect is less than that of formocresol and camphorated parachlorophenol. It is less irritating to the tissue.

Camphorated Monochlorophenol (CMCP)
- Camphorated monochlorophenol consists of 2 parts of parachlorophenol and 3 parts of gum camphor (p-chlorophenol 35%, camphor 65%).
- Camphor serves as a vehicle and diluent and reduces the irritating effect of pure PCP. It also prolongs the antimicrobial effect. It is used in the form of vapour forming intracanal medicaments. The vapours can pass through the apical foramen.

Glutaraldehyde
- Glutaraldehyde is a colorless oil slightly soluble in water. It is a strong disinfectant and fixative. The antimicrobial action of glutaraldehyde is bacteriostatic in nature. The recommended concentration is 2%.
Formaldehyde Based (N2)
- N2 was introduced by Sargenti. It is a compound containing paraformaldehyde as the primary ingredient. It also contains Phenylmercuric borate, eugenol, and additional ingredients like lead, corticosteroid and antibiotics. It is claimed to be both intracanal medicament and sealer. The antibacterial effect of N2 is short-lived.
Antibiotics
Polyantibiotic Pastes – PBSC And PBSN
- Polyantibiotic pastes were first introduced in 1951 by Grossman who was considered as the father of Endodontics. Grossman’s paste was also known as PBSC which stood for penicillin, bacitracin, streptomycin and caprylate sodium suspended in silicone oil. Subsequently the antifungal agent caprylate sodium was replaced by Nystatin, i.e. PBSN.
Composition
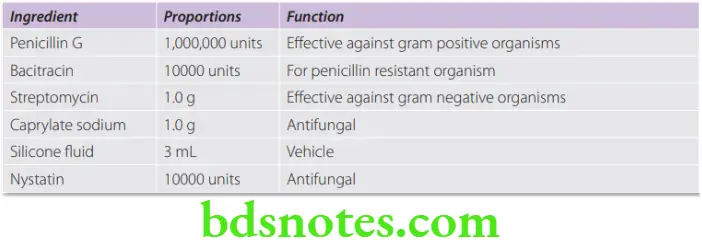

- They are available in a paste form that may be injected into root canals impregnated on paper points.
Drawbacks and concerns
- Polyantibiotic paste showed therapeutic potential, but owing to the drawbacks including ineffectiveness against anaerobic species and allergic reactions, the Food and Drug Administration (FDA) prohibited PBSC for endodontic use in 1975. Fears of development of resistant organisms was another concern. Antibiotics for use in root canal therapy should be carefully justified in order to avoid development of bacterial resistance.
Sulfonamides
- Sulfonilanide and sulfanizole are used as medicaments by mixing with sterile distilled water or by placing a moistened paper point into a fluffed jar containing the powder. This medication is suggested for use as intracanal medicament in acute periapical abscess.
- Disadvantage Yellowish tooth discoloration has been reported after use.
Corticosteroid – Antibiotic Combinations
- These medicaments are highly effective in the treatment of over instrumentation. They must be placed in the inflamed tissue by a paper point or reamer to be effective. They are more effective in vital pulps than the necrotic pulp tissue. The steroid constituent reduces the periapical inflammation and gives instant relief of pain. The antibiotic constituents are present so that overgrowth of microorganisms will be prevented. An example of this category is the Ledermix paste developed by Schroeder and Triadan in 1960 which contains an antibiotic demeclocycline—HCl (3.2%) and a corticosteroid, triamcinolone acetonide (1%), in a polyethylene glycol base. Another example is the Odontopaste released in February 2008 (zinc oxide-based root canal paste with 5% clindamycin hydrochloride and 1% triamcinolone acetonide).
Halogens
- Sodium hypochlorite is also used as an intracanal medicament (ICM). Chlorine is the active ingredient in NaOCl. Sodium hypochlorite reacts rapidly with organic matter, and hence the longevity of its antimicrobial effect is questionable.
- Disadvantage Toxic to the periapical tissues.
Aminoacridine
- It is a mild antiseptic. It works by inhibiting bacterial protein synthesis.
Quaternary Ammonium Compounds
Chloramine
- It is a chlorine compound (NH2Cl) used in concentration of 5%. It has good antimicrobial qualities. It remains stable for a long period of time.
Iodine
- Iodine is highly reactive combines with proteins and forms salts which probably destroys micro- organisms. Iodine-potassium iodide has a relatively high antibacterial effect and relatively low toxicity.
Disadvantage
- It may cause staining of the tooth.
- Allergic reaction.
Calcium Hydroxide
- Calcium hydroxide was introduced by Herman in 1920. It is one of the commonly used ICMs (Intracanal medicaments). It is a broad spectrum antimicrobial agent. Its antiseptic action probably relates to its high pH and its leaching action on necrotic pulp tissue. It is best used in ‘weeping canals’, where there is a constant clear or reddish exudate associated with large periapical lesion. In such cases calcium hydroxide is an excellent medicament to be used.
Chlorhexidine (CHX) Gluconate

- Chlorhexidine (CHX) (2% gel) is also shown to be an excellent intra canal medicament. It is broad spectrum antimicrobial agent. It can be used alone in gel form or mixed with Ca(OH)2. CHX gel provides antimicrobial activity for up to 21 days after contamination. When it is used in combination with Ca(OH)2, the antimicrobial activity of this mixture is greater than the combination of Ca(OH)2 and saline.

Leave a Reply