Electrochemical Properties Of Materials
Except for a few, pure metals do not occur naturally. They occur in the form of minerals such as oxides and sulfides and these have to be refined to produce the pure metal. Most pure metals attempt to reconvert to the combined state. The process by which this takes place is called corrosion.
One of the primary requisites of any metal that is to be used in the mouth is that it must not produce corrosion products that will be harmful to the body. The mouth is moist and continually subjected to fluctuations in temperature. The foods and liquids ingested have wide range of pH. All these factors make the mouth an extremely favorable environment for corrosion.
Read And Learn More: Basic Dental Materials Notes
Definitions
Tarnish Definition
Tarnish is a surface discoloration on a metal or even a slight loss or alteration of the surface finish or lustre.
Tarnish generally occurs in the oral cavity due to
- Formation of hard and soft deposits on the surface of the restoration, e.g. calculus, mucin and plaque.
- Pigment producing bacteria, produce stains.
- Formation of thin films of oxides, sulfides or chlorides.
Tarnish is often the forerunner of corrosion.
Passivation Definition
- In certain cases, the oxide film can also be protective in nature. For example, chromium alloys (used in dental castings) are protected from corrosion by the formation of an oxide layer on its surface which protects the metal against any further corrosion. This is known as passivation. Another example is titanium.
Corrosion
- It is not a surface discoloration but actual deterioration of a metal by reaction with the environment. It can be defined as the deterioration of metals by chemical interaction with their environment.
- Most metals exist in their stable oxide state in nature except for some of the noble metals like gold. Metals are refined from these natural ores to produce the pure metals and alloys. However, the pure state of metals is unstable. Corrosion is a natural process, which converts refined metal to their more stable forms.
- In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion. This type of damage typically produces oxides or salts of the original metal.
- However element other than oxygen also can cause corrosion particularly in the oral environment. Water, oxygen, chloride ions, sulfides like hydrogen sulfide or ammonium sulfide contribute to corrosion attack in the oral cavity. Various acids such as phosphoric, acetic and lactic are also present. Among the specific ions responsible for corrosion, oxygen and chloride have been implicated in amalgam corrosion both at the tooth interface and within the body of amalgam. Sulfide has been implicated in the corrosion of silver containing casting alloys.
- Corrosion degrades the useful properties of materials and structures including strength and appearance. In due course, it may lead to rapid mechanical failure of the structure.
Electromotive Force Series (EMF) Definition
- The EMF series is a classification of elements in the order of their dissolution tendencies. That is, if two metals are immersed in an electrolyte and are connected by an electrical conductor, an electric couple is formed. The metal that gives up its electrons and ionizes is called the anode. In the EMF series, hydrogen has been used as the standard electrode to which other metals have been compared. Hydrogen has been given the value zero in the EMF series.
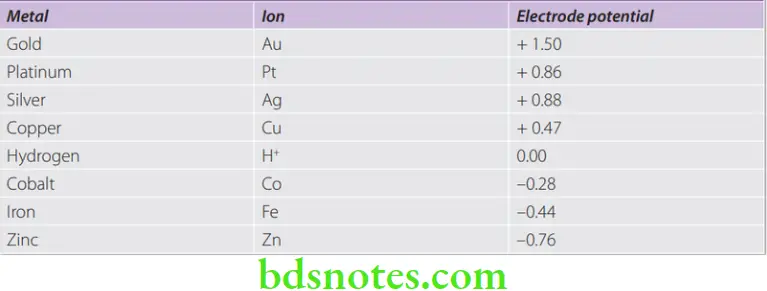
- The metal with lowest electrode potential corrodes. Also the more active metal corrodes (anode) and the more noble metal becomes the cathode.
Classification Of Corrosion
Corrosion can be classified as
- Chemical or dry corrosion
- Electrochemical or wet corrosion
Chemical or Dry Corrosion
The metal reacts to form oxides and sulfides in the absence of electrolytes.
- Example Formation of Ag2S in dental alloys containing silver.
- Oxidation of alloy particles in dental amalgam.
Electrolytic or Electrochemical or Wet Corrosion
- This requires the presence of water or other fluid electrolytes. There is a formation of free electrons and the electrolyte provides the pathway for the transport of electrons. An electrolytic cell is as follows:
⇒ \(\mathrm{M}^3 \rightarrow \mathrm{M}^{+}+\mathrm{e}^{-}\)
- The anode is the surface where positive ions are formed. This metal surface corrodes since there is a loss of electrons. This reaction is sometimes referred to as an oxidation reaction.
⇒ \(\mathrm{M}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{M}^3\)
⇒ \(2 \mathrm{H}^{+}+2 \mathrm{e}^{-} \rightarrow \mathrm{H}_2\)
⇒ \(2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2+4 \mathrm{e}^{-} \rightarrow 4(\mathrm{OH})^{-}\)
- At the cathode, a reaction must occur that will consume the free electrons produced at the anode. Reactions 2, 3, and 4 occur at the cathode and are referred to as reduction reactions. Hence, the anode loses electrons and the cathode consumes. The surface of the anode corrodes due to the loss of electrons.
Types Of Electrolytic Corrosion
Galvanic Corrosion
- Saliva with its salts provides a weak electrolyte. Galvanic corrosion occurs when dissimilar metals lie in direct physical contact with each other.
- If a gold restoration comes in contact with an amalgam restoration, the amalgam forms the anode and starts corroding. The electric couple (500 millivolts) created when the two restorations touch, causes sharp pain called ‘galvanic shock’.
- It usually occurs immediately after insertion and can be minimized by painting a varnish on the surface of the amalgam restoration. However, the best precaution is to avoid dissimilar metals in contact. Another variation of galvanic corrosion can occur even in a lone-standing restoration.
- Note Seldom there is no one type of corrosion found alone, generally two or more act simultaneously and thus aggravate the problem.

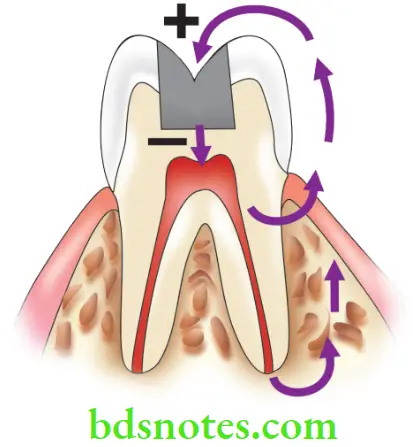
Heterogeneous Compositions
This kind of corrosion occurs within the structure of the restoration itself. Heterogeneous (mixed) compositions can cause galvanic corrosion.
- When an alloy containing eutectic is immersed in an electrolyte the metallic grains with the lower electrode potential are attacked and corrosion results.
- In a cored structure differences in the composition within the alloy grains are found. Thus a part of a grain can be an anode and a part, a cathode. Homogenization improves the corrosion resistance of the alloy.
- In metals or alloys, the grain boundaries may act as anodes and the interior of the grain as the cathode.
- Solder joints may also corrode due to the nonhomogeneous composition.
- Impurities in any alloy enhance corrosion.
Stress Corrosion
- A metal that has been stressed by cold working becomes more reactive at the site of maximum stress. If stressed and unstressed metals are in contact with an electrolyte, the stressed metal will become the anode of a galvanic cell and will corrode. For example, if an orthodontic wire has been cold worked, stress corrosion may occur and cause the wire to break.
Concentration Cell Corrosion or Crevice Corrosion
- Electrolyte concentration cell In a metallic restoration that is partly covered by food debris, the composition of the electrolyte under the debris will differ from that of saliva and this can contribute to the corrosion of the restoration.

- Oxygen concentration cell Differences in oxygen tension between parts of the same restoration cause corrosion of the restoration. Greater corrosion occurs in the part of the restoration having a lower concentration of oxygen.
Factors affecting corrosion of restorations in the mouth
Corrosion of dental restorations in the mouth is influenced by
- Diet
- Drug
- Smoking
- Bacterial activity
- Oral hygiene and habits
Protection Against Corrosion
Passivation
- Certain metals readily form a strong adherent oxide film on their surface, protecting them from corrosion. Such metals are said to be passive. Chromium, titanium, and aluminum are examples of such metals.
- Adding more than 12% Cr to iron or cobalt produces a chromic oxide layer on the surface of stainless steel or cobalt-chromium alloys which is highly corrosion resistant. Since this film is passive to oxidative chemical attack, their formation is called passivation.
Increasing Noble Metal Content
- Alloys with a noble metal content below 65% may tarnish. So it has been suggested that at least 50% of the atoms in a dental alloy should be gold, platinum or palladium to ensure against corrosion. Noble metals resist corrosion because their EMF is positive about any of the common reduction reactions found in the oral environment.
Polishing
- Polishing metallic restorations like amalgam and cast metal to a high luster minimizes corrosion. The patient should also maintain good oral hygiene.
Other Methods
- Dissimilar metal restorations should be avoided. Avoid using a high mercury-containing amalgam as it is more susceptible to corrosion. Mercury tarnishes gold, thus, care must be taken to protect gold ornaments worn by the operator, assistant, or patient.

Leave a Reply