Drugs In Dentistry
Question 1. Describe Astringents.
Or
Give Description Of Astringents.
Or
Write Briefl On Astringent Drugs.
Or
Write On Astringents (Name, Actions, And Uses).
Or
Write Of Astringents Definition, Names, And Uses.
Or
Write Short Note On Astringents.
Answer:
Astringents are those substances that cause precipitation and do not cause penetration to cells, thus affecting the superficial layer only. Astringents are divided into the following categories. Examples:
1. Vegetable astringents:
- Catechu: It is used for mouthwash.
- Tannic acid: At a concentration of 3.5% it is used as mummifying agent, obtundent, and mouthwash.
Read And Learn More: Pharmacology Question And Answers
2. Alcohol: At 50 to 90% concentration ethanol and methanol are the perfect astringents.
3. Mineral astringents: These are the heavy metal ions that are used as antiseptics and astringents. They are:
- Aluminum salts: Alum and aluminum acetate is used. Alum hardens the gum if they are inflamed and ulcerated. Since it is acidic, it can damage the enamel. Aluminum acetate is less irritating.
- Zinc salts: These are zinc chloride, zinc sulfate, and zinc oxide. They have both astringent and antiseptic properties. They do not cause staining of teeth
- Ferric chloride: It stains the teeth and damages enamel so it is not used as an astringent these days.
- Copper sulfate: At a concentration of 0.5 to 2% it is used as an astringent to treat gum ulcers.
Astringents Actions
- They toughen the tissue surfaces making them mechanically stronger and decreasing exudation.
- It causes hemostasis and reduces blood flow.
- They resist the penetration of bacteria and form a protective covering over underlying tissues.
Question 2. Describe Mummifying Agents.
Or
Write A Short Note On Mummifying Agents.
Answer:
Mummifying agents are used for drying and hardening tissues of the root canals and pulp to protect them from infection and to maintain an aseptic condition.
- Mummifying agents are used when pulp removal is not possible and the contents of a root canal are not removed completely.
- The mummifying agents are:
- Iodoform: It acts by the liberation of iodine. It is made into a paste with eugenol, phenol, tannic acid, cinnamon oil, and glycerol for use as a mummifying agent.
- Liquid formaldehyde: It is used with zinc oxide and glycerine with local anesthetics like lignocaine to harden the tissues. It should be diluted 3 to 4
times with water before use. - Paraform: It acts by releasing formaldehyde very slowly and is used in combination with zinc oxide and glycerine or other combination like zinc oxide, cresol, and zinc sulfate.
- Cresol, tannic acid, and ammonia silver nitrate: These are the other substances that can be used as mummifying agents.
Mummifying Agents Disadvantage
The major disadvantage of the mummifying agent was dead tooth pulp is retained, albeit in a dry and hard condition, but the chances of future infection or inflammation should not be ruled out.
Question 3. Give Description Of Mouthwashes.
Or
Write A Short Note On Mouthwashes.
Or
Give a brief account of drugs in oral hygiene.
Answer:
They are basically deodorants and antiseptics.
- Mouthwashes also help in cleansing by removing water-soluble substances, and by removing loose debris from surfaces between the teeth and from the oral cavity.
- Mouthwashes are mainly alcoholic or hydroalcoholic solutions as they are used in the oral cavity which is diluted if necessary.
- However, there can be mouthwash as solid, powder, or concentrated products which are to be dissolved or diluted with water before being used as a mouthwash.
- The components of mouthwash are:
- Antiseptics, i.e. phenol and its derivatives
- Astringents, i.e. zinc chloride and zinc acetate
- Deodorizing agents, i.e. chlorophyllin
- Drug extracts, i.e. tincture of myrrh
- Flavors, i.e. peppermint oil, menthol, and clove oil
- Surfactant
- Sweeteners, i.e. saccharine
- Colors: Erythrosine and caffeine
- Vehicles, i.e. alcohol alone or in combination with water in definite proportion.
Types Of Mouthwashes
- Mechanical cleansing mouthwash
- Plaque inhibitory: Inhibits plaque formation
- Antiseptic mouthwash: Used in the treatment of acute ulcerative gingivitis
- Obtundent mouthwash: For sensitive oral lesions
- Detergent mouthwash: For cleansing and deodorizing action.
Mouthwashes Uses
- In sensitive oral lesions
- In halitosis
- Soreness under dentures
- Postoperative and other bedridden patients for maintaining oral hygiene
- In stomatitis
- Post-disimpaction of the tooth.
Question 4. Describe Dentifrices.
Or
Give A Brief Account Of Dentifrices.
Or
Write Briefl On Dentifrices.
Or
Write On Dentifrices, Definition, Names, And Uses.
Or
Write Short Note On Dentifrices.
Or
Write In Short On Dentifrices.
Answer:
Dentifrices are the preparations used for cleansing the surfaces of teeth and keeping them shiny to preserve the health of teeth and gums.
- Dentifrices may also be expected to inhibit the formation of unpleasant odors and freshen the breadth.
- Regular use of dentifrices helps to prevent the occurrence of tooth decay.
- Dentifrices can either be simple cleansing or also be therapeutic dentifrices.
- These dentifrices, i.e. toothpaste and tooth powders consist of:
- Mild abrasives and polishing materials: These materials are known as cleansing materials. Abrasive is the main constituent of toothpaste and powders, for example, calcium carbonate, aluminum sulfate, and magnesium trisilicate.
- Detergent and foaming materials: The detergents and foaming agents cause cleansing by lowering surface tension. The detergents help in the wetting dispersion of powdered materials in paste, for example, sodium lauryl sulfate, and magnesium lauryl sulfate.
- Humectants: They prevent the drying of the product and impart plasticity character to paste. For example, glycerol, sorbitol, and propylene glycol.
- Binding agents: Various hydrocolloids are used in toothpaste to maintain consistency, for example, gum tragacanth.
- Sweetening materials: It provides a sweet taste, for Example. Saccharine.
- Flavors: They produce long-term effects of tooth powder and toothpaste in the mouth, for example, peppermint oil.
- Coloring agent: Many of the dentifrices are white, but some are brightly colored blue, green, or cherry red to make them attractive by adding methylene blue chlorophyll and liquor ruler or other permitted colors.
Medicated Dentifrices
Certain medications are added to the toothpaste and toothpowders so as to empower them with prophylactic/therapeutic activity against specific dental conditions. These are:
- Fluoride: Sodium mono fluorophosphate or sodium fluoride for caries prevention. Most toothpastes contain fluoride.
- Antiseptics: Chlorhexidine, triclosan, or benzalkonium chloride for prevention and treatment of dental plaque. A copolymer is often included with triclosan to prolong its substantivity.
- Desensitizing agents: Potassium nitrate or strontium chloride are mostly added to treat dentine sensitivity.
- Bleaching agents: Carbamide peroxide is the most common bleaching agent added to stain-removing dentifrices.
Dentifrices Uses
- Maintains oral hygiene
- Helps in the prevention of dental caries
- Prevents gingivitis
- Prevents periodontal diseases
- Prevents halitosis
- Removes stains from the teeth
- Helps in suppressing hypersensitivity of the teeth.
- They are also used for the cosmetic whitening of teeth.
Question 5. Comment On Obtundents.
Or
Give A Brief Account Of Obtundent Drugs.
Or
Write A Short Note On Obtundent.
Answer:
- Obtundents are agents which are used to diminish or eliminate the dentin sensitivity to make the excavation procedure painless.
- Obtundents act by:
- Stimulation followed by desensitization of nerve endings: Clove oil, thymol, menthol, camphor, and phenol.
- Astringent action: Stannous chloride, zinc chloride, paraformaldehyde.
Obtundents Mechanism Of Action
- Stimulation followed by desensitization of nerve endings: These obtundents have counter-irritant properties and produce relative numbness due to desensitization of sensory nerves lasting for one to few hours.
- Astringent action: They precipitate surface proteins and interfere with the function of pain receptors. Pain relieving action is mild.
Different Obtundents In Brief
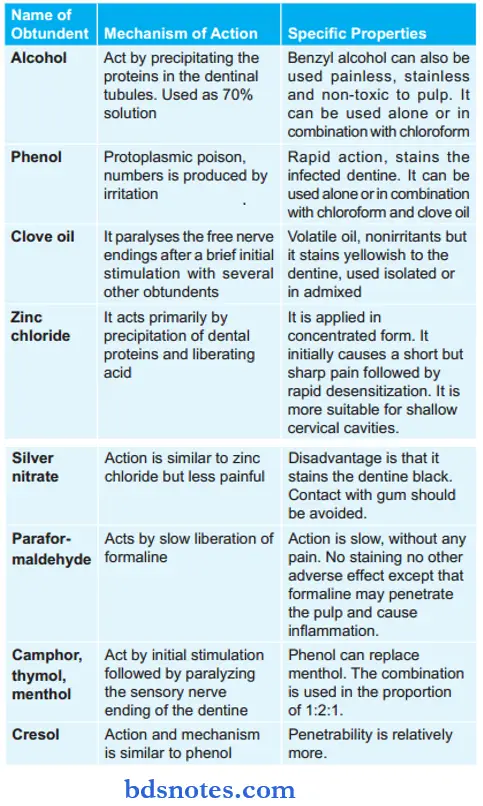
The above drugs mentioned are replaced. They are replaced by local anesthetics, for example, lignocaine. Lignocaine giving topically is mostly used for pain excavation.
Obtundents Uses
- To make pulp excavation painless.
- Reduce pain in alveolar osteitis.
Obtundents Disadvantage
Irritant obtundent may shrink the pulp.
Question 6. Write Briefl On Drugs In Dental Caries.
Or
Give A Brief Account Of Dental Caries Prevention.
Or
Describe The Drug Treatment Of Dental Caries.
Or
Write Short Note On Anticaries Agents.
Answer:
Dental caries is an infectious microbiologic disease of teeth that results in localized dissolution and destruction of calcified tissues.
Drugs In Dental Caries
Drugs used in dental caries are known as anticaries drugs. Anticaries drugs are:
- Fluoride: Makes tooth more resistant to caries and has weak antibacterial action.
- Antiplaque agents: Mainly chlorhexidine and triclosan reduce the population of cariogenic bacteria.
Dental Caries Fluoride
Fluoride is a halogen and is highly reactive and electronegative
Dental Caries Mechanism Of Action
- Hydroxyapatite crystals lead to the hardness of tooth enamel. But the hydroxyapatite crystals are readily dissolved by the action of acids over a period of time. Fluoride radical is highly reactive, so it exchanges with hydroxyl radical and leads to the formation of fluorapatite. Fluorapatite is more compact, and harder, and is a less acid-labile substance than hydroxyapatite. This leads to teeth becoming more caries-resistant.
- Fluoride also leads to remineralization of enamel which is attacked by the acid. Free fluoride ion release from fluorapatite by the action of acid raises local fluoride ion concentration and facilitates remineralization of damaged enamel.
- Fluoride inhibits plaque formation and also reduces anaerobic glycolysis and lactic acid formation within the formed plaque.
Application Of Fluoride
Systemic Administration
Fluoridation via Water Supply
- 0.5–l ppm, i.e. 1 part of fluorine to 1 million parts of water is considered adequate for prophylaxis purposes. Fluoridation of drinking water is the most effective measure in preventing dental caries if consumed prior to the eruption of the permanent teeth. Several large-scale studies have established the beneficial effects of optimum fluoridation of communal water supplies in preventing caries. More than 1–2 ppm results in toxicity, and causes dental fluorosis.
- Adequate prophylaxis can also be achieved by supplementing fluorine in the form of sodium tablets in the diet. It is suggested that using one tablet a day during the period of tooth development for nursing mothers and children up to the completion of calcification of third molars.
Fluoridation via Salt
In Europe, table salt is fortified with 200 to 350 mg/Kg of salt in deficient areas.
Sodium Fluoride Tablets/Lozenges/Drops
Infants and children in areas where there is no mass fluoridation are protected from a high incidence of dental caries by giving sodium fluoride tablets, lozenges, and drops till the age of 16 years. Lozenges and drops also provide a local application to the teeth.
Topical Application
- Fluoridated toothpaste: Common salt added to fluoridated toothpaste is sodium mono fluorophosphate 0.76%. Other salts used have some limitations. Care should be taken to avoid ingestion of fluoridated toothpaste
- Fluoride mouth rinses: Sodium fluoride or stannous fluoride is used as a daily mouth rinse in susceptible individuals to prevent dental caries. The rinse solution is held in the mouth for 1 to 3 min and is swished around. It is now discarded and food/drink are avoided for 30 min to minimize washing away of fluoride in contact with teeth.
- Professionally applied fluoride: This is done by the dentist at long intervals.
- Acidulated phosphate fluoride (APF): Formulated as a gel or solution it consists of 1.23% fluoride and 0.1M orthophosphoric acid. Ph is adjusted to 3. Acidic medium enhances fluoride diffusion into the enamel while orthophosphoric acid prevents enamel dissolution. Applications are repeated at 6 months of interval and the optimal duration of each application is 4 min.
- Fluoride varnish: They are non-aqueous preparations that are not washed off by saliva and are retained on teeth for longer periods. A 2% sodium fluoride lacquer in resin base or a polyurethane varnish containing 0.7% fluoride is painted on teeth or applied to the oral cavity.
Dental Caries Anti-Plaque Agents
The two most commonly used antiplaque agents in dental caries are:
- Chlorhexidine: It is used as a mouth rinse or gel and reduces the population of S. mutants and other cariogenic bacteria. The risk of caries occurrence is reduced but regular use causes staining of teeth.
- Triclosan: Since it is non-irritant, tasteless, and nonstaining, it is included with active ingredients in many anticaries and antiplaque toothpaste and gels.
- Chewing of sucrose-free polyol gum for 10 to 20 min after meals can be used in children and adults at high risk of developing caries. Xylitol has antimicrobial effects and inhibits acid production in the oral cavity.
- A 1:1 mixture of chlorhexidine/thymol varnish is effective in the prevention of root caries in adults and the elderly.
- Calcium and phosphate in toothpaste or mouth rinse will increase the concentration of these ions in the oral cavity and improve remineralization.
Question 7. Write Briefl On Drug Against Oral Ulcers.
Answer:
An oral ulcer is a small white spot on the mucous membrane of the mouth.
Drugs Against Oral Ulcer
- Topical corticosteroid: Triamcinolone dental paste is used in oral ulcers.
- Local analgesics: Drugs used are 5% Lignocaine ointment.
- Simple mouthwash: Thymol glycerine as mouthwash relieves pain in ulcers.
- Antiseptic mouthwash: Chlorhexidine as mouthwash causes the healing of ulcers.
- Gelatin paste is used for mechanical protection.
Question 8. Write A Short Note On Drugs In The Treatment Of Gingivitis.
Or
Write Down The Drug Treatment Of Gingivitis.
Or
Write Short Note On The Treatment Of Gingivitis.
Answer:
Inflammation of the gingiva is known as gingivitis.
- It may occur in acute, subacute, or chronic forms.
- The causes of gingivitis are:
- Microorganism
- Calculus
- Food impaction and general oral neglect.
- Faulty or irritating restoration appliances.
- Mouth breathing
- Tooth malabsorption.
Gingivitis Treatment
- Ornidazole topically is applied for 4 to 5 days TID.
- Tab. Ibuprofen 400 mg orally TID after food for 3 days
- Gumpaint (Tannic acid 2% + Zinc chloride 1% + Cetrimide 0.1%) is applied for 4 to 5 days TID.
- Mouthwash containing chlorhexidine should be swished in the mouth for 1 min and then split. It is used for 4 to 5 days BD. After using mouthwash nothing should be eaten or drunk for 1 hour.
Question 9. Write The Drug Treatment Of Oral Thrush.
Or
Give A Brief Account Of Drugs In Oral Thrush.
Answer:
Oral thrush is also known as candidiasis.
- It is an infection caused by a fungus known as Candida albicans.
Oral Thrush Treatment
Clotrimazole troche of 10 mg which is allowed to dissolve in mouth QDS for 14 days.
Or
Nystatin suspension of 4 to 6 ml is swished and swallowed QDS for 14 days.
Or
Cap. Fluconazole 200 mg on first day and 100 mg a day for 14 days.
Question 10. Write A Brief Account Of Drugs In Gingival Abscess.
Answer:
- Drugs In Gingival Abscess
- Tab metronidazole 400 mg orally TID for 7 days.
- Cap amoxicillin + clavulanic acid 625 mg TID for 7 days.
- Tab Ibuprofen 400 mg TDS for 3 days.
Treatment Of Gingival Abscess
- After topical anesthesia, i.e. lignocaine is applied the fluctuant area of the lesion is anesthetized with a blade, and the incision is gently widened to permit drainage.
- The area is cleaned with warm water and covered with an adrenaline-containing gauge pad. After the bleeding is stopped patient is instructed to rinse every two hours with mouthwash.
Question 11. Describe Drug Therapy Of Toothache.
Answer:
Drugs To Be Used In Toothache
NSAIDs are the main drugs for the management of toothache. The nature of pain, risk factors, and individual preferences are to be considered while prescribing an NSAID.
- For mild to moderate pain with little inflammation: Paracetamol or low-dose ibuprofen.
- Postextraction or acute short lasting pain: Ketorolac, diclofenac and nimesulide.
- Gastric intolerance or peptic ulcer patients: Paracetamol or selective COX 2 inhibitor.
- During toothache, if the patient is with history of asthma or anaphylactic reaction to aspirin/other NSAIDs: Nimesulide is given.
- If the toothache is present in the pediatric patient: Paracetamol, Ibuprofen, and Naproxen are given.
- If toothache during pregnancy paracetamol is the safest drug to be given.
- If the toothache is in diabetics, CHF, and in epileptics, the physician should be consulted before giving analgesia.
Question 12. Write On Drugs Used In Xerostomia.
Answer:
Xerostomia means dry mouth.
- It is caused by damage/disease to the salivary glands and by the administration of drugs with antimuscarinic side effects, i.e. antipsychotics, antidepressants, antispasmodics, anticholinergics, and clonidine.
- Xerostomia is treated in the following manner:
- Pilocarpine nitrate tablets of 5 mg are used for TDS with or after meals.
- Artificial saliva is of neutral pH and contains electrolytes corresponding approximately to saliva.
- Frequent sips of cold drinks, sucking pieces of ice, or sugar-free fruit drops.
Question 13. Answer The Following Questions By Giving Reasons:
1. Enumerate Drugs Which Cause Gingival Hypertrophy. Describe The Pharmacology Of Anyone.
2. Enumerate Drugs Used In Dental Abscess And Describe The Pharmacology Of Anyone.
Answer:
1. The drugs which cause gingival hypertrophy are:
- Antiepileptic drugs, i.e. phenytoin
- Calcium channel blockers, i.e. diltiazem, verapamil, rifampin.
- Immunosuppressants: Cyclosporine.
2. Dental abscess is of two types, i.e.
- Periodontal abscess
- Periapical abscess
Drugs used in periodontal abscess: Tetracycline, doxycycline, metronidazole tablets, ointments, and mouth rinses such as chlorhexidine gluconate.
Drugs used in periapical abscess: Oflxacin+ ornidazole combination, ciprofloxacin + tinidazole combination, amoxicillin + clavulanic acid.
Question 14. Describe Pharmacotherapy Of Oral Ulcers.
Answer:
Pharmacotherapy Of Oral Ulcers In Mild Cases
- Topical protective emollient base.
- Topical tetracycline mouthwash. Use four times daily for 5 to 7 days.
- Topical corticosteroid preparation, i.e. triamcinolone acetonide.
- Replacement therapy with vitamin B12, ferritin, folate, and iron.
- Topical application of tetracycline. Chlorhexidine mouthwash.
Pharmacotherapy Of Oral Ulcers In Severe Cases
- Fluocinolone gel, clobetasol cream or beclomethasone spray.
- Injection of corticosteroid directly into the lesion.
- Chlortetracycline as mouth rinses to be flushed over the affected region.
- In some cases, dapsone or thalidomide can be used.
- Interferon alpha, nicotinic tablets, and colchicine can be used.
Question 15. Enumerate The Drugs Used In Dental Plaque And Management Of Floride Toxicity.
Answer:
Drugs Used In Dental Plaque
Drugs used in dental plaque are known as antiplaque agents. Antiplaque agents are:
- Chlorhexidine
- Quaternary ammonium antiseptics: Cetylpyridinium chloride, benzalkonium chloride
- Phenols: Triclosan, Listerine
- Oxygenating agents: Hydrogen peroxide, sodium perborate
- Zinc citrate
- Stannous fluoride
- Sanguinarine.
Management Of Fluoride Toxicity
Prehospital care: Place patients with known significant ingestion of fluoride on a cardiac monitor and initiate an IV line.
Administer calcium IV to patients who present with cardiac dysrhythmias.
Emergency Department Care
- Provide cardiac monitoring.
- Hypocalcemia may be detected.
- Perform gastric aspiration and lavage. Small-bore nasogastric tube aspiration, followed by lavage, is recommended because of the potential severity of this ingestion and the ineffective absorption of fluoride by activated charcoal. Lavage with milk or a solution containing calcium or magnesium hydroxide (For example. milk of magnesia) is theoretically attractive but has not been proven beneficial. Some recommend lavaging with 1–5% calcium chloride solution to bind fluoride in the stomach.
- Gastric aspiration and lavage are most effective when instituted within 1 hour of ingestion.
- Administer milk, calcium carbonate, and aluminum and magnesium-based acids (For example. hydroxides) tobindfloride.
- Activated charcoal is not helpful. Fluoride does not bind to charcoal. Activated charcoal is still recommended for those with intentional ingestions when a polysubstance overdose is possible.
- Correct calcium deficiencies with IV calcium chloride.
Question 16. Write A Short Note On Dentifrices Names And Actions.
Answer:
Dentifrices are the preparations used for cleansing the surfaces of teeth and keeping them shiny to preserve the health of teeth and gums.
- Vehicles: For chemotherapeutic agents to inhibit plaque, calculus, caries, or root hypersensitivity.
- Pyrophosphate-containing dentifrices: Tartar control interferes with crystal formation in calculus and does not affect fluoride ions in paste or increased tooth sensitivity.
- Fluoride-containing dentifrices: Dentifrices contain stannous fluoride in combination with calcium pyrophosphate as the cleansing and polishing system which decreases the incidence of dental caries. Some of these dentifrices contain sodium fluoride. (Crest or Crest Tartar Control)
- Others containing sodium mono fluorophosphate: These are ADA-specified dentifrices (Aim, Aquafresh, Colgate, Masleans).
Question 17. Write In Short Note On Disclosing Agents.
Answer:
Dyes used to facilitate clear visualization of dental plaque are called disclosing agents.
By staining the bacterial plaque deeply, they increase the contrast between the plaque and the gums.
Purpose Of Selection Of Dye As Disclosing Agent
Dyes selected for the purpose are those which:
- Have a higher affinity for bacterial plaque than for oral mucosa/teeth.
- They are not bad tasting, irritating or toxic.
- They diffuse uniformly and stain all supragingival plaque.
- These are easily washed off by rinsing after plaque removal.
Various Dyes Used As Disclosing Agents
Dyes used as disclosing agents are:
- Erythrosine: It is the most commonly used disclosing agent. This red dye is bland tasting, nontoxic and stains plaque deeply, but the gums and oral mucosa also take light stains. The residual stain goes after repeated rinsing.
- Fluorescein: It is a yellow dye that fluoresces under ultraviolet light; is selectively taken up by the bacterial plaque, but not by oral soft tissue. As such, after rinsing with an aqueous solution of fluorescein, the plaque can be demarcated clearly but needs ultraviolet light. However, it is nonirritating and nontoxic.
- Two-tone dye: It is used to differentiate the older and thicker part of plaque from the thinner, newer plaque. A mixture of red and green dye is used; the mature plaque appears blue while the fresh plaque appears red.
Application Of Disclosing Agent
Disclosing agents may be applied either by rinsing the mouth with a dilute solution of the dye, or it may be painted on the teeth with a brush/cotton swab. If the dye is available in tablet form, it may be chewed, swished around in the mouth followed by rinsing.
Question 18. Write In Short On Desensitizing Agents.
Answer:
Desensitizing Agents
Desensitizing agents are those applied to the teeth to mitigate dentine hypersensitivity, i.e. shooting pain triggered from sensitive teeth by thermal (hot and cold), mechanical (touch, chewing, a blast of air), or chemical (sour and sweet food) stimuli.
Dentine may get exposed to external stimuli due to enamel damage caused by chewing hard substances, age-related tooth addition, erosion by acidic food at the crown; or due to denudation of root as a result of the gingival recession of old age, faulty brushing, periodontal disease, etc.
Desensitizing Agents’ Mechanism Of Action
Dentine is traversed by numerous fie flid-filed dentinal tubules.
When these tubules are exposed, mechanical and thermal stimuli cause abnormal perturbations of the fluid in the tubules and activate the nerve endings at their inner mouth or in the pulp.
While soluble chemicals (acids/sugars in food) diffuse through the tubules and act on the sensory nerves—all producing sharp pain.
The desensitizing agents aim to interrupt this pain-inducing process by either creating a plug in the dentinal tubules, sealing their mouth at the tooth surface, or modulating the generation of painful nerve impulses.
Various Desensitizing Agents
The commonly used desensitizing agents are:
- Potassium nitrate: At a concentration of 5%, it is the most frequently included active ingredient of desensitizing toothpaste. The paste is to be applied on the sensitive teeth and left in place for 5 minutes before brushing lightly and then rinsing it off This is repeated 2-3 times daily. Potassium nitrate is believed to obliterate the dentinal tubules by precipitation. It may also dampen the pain-inducing nerve impulses.
- Strontium chloride: It is an alkali-earth metal salt that precipitates proteins in the dentinal tubular fluid and thus tends to limit/obstruct the easy displacement of fluid by pain-inducing stimuli. Calcification of the bony component of the tooth is believed to be hastened by strontium ions providing another mechanism of desensitizing action.
- Potassium oxalate: It diffuses into the dentinal tubules, and reacts with ionic calcium in the fluid there to produce calcium oxalate which is insoluble in deposits as crystals. These crystals hinder fluid movement in the tubules induced by external stimuli, thereby lessening the pain.
- Fluoride: Fluoride compounds like sodium mono fluorophosphate, and sodium/stannous fluoride are included in many multi-ingredient desensitizing toothpastes. These may react with calcium and produce calcium fluoride crystals in the dentinal tubules. Stannous fluoride may deposit five layers of tin particles in the tubules creating a partial obstruction. In the long-term fluoride ion accelerates secondary dentine formation which may reinforce the tubules and reduce dentine sensitivity.
- Formaldehyde: At a concentration of 1-1.5%, formaldehyde is a weak desensitizing agent. Its most prominent action precipitation of proteins. Such action within the dentinal tubules may underlie its desensitizing property. However, it has a disagreeable taste and smell and is not favored now.
- Dentine bonding agents: Certain acrylic bonding agents i.e. hydroxy ethyl methacrylate, some resins, composites, varnishes, etc. have been developed which can be burnished on the exposed root or sensitive part of the crown to seal the external openings of the exposed dentinal tubules. After suitable preparation of the sensitive tooth and the use of primers, the bonding agent is applied and allowed to dry. A long-lasting bonding with dentine occurs rapidly so that stimuli that induced pain earlier are blocked from reaching the pulpal nerve endings.
Question 19. Write Notes On Florides.
Answer:
Fluorine is a halogen. Being a highly electronegative element, it is highly reactive.
- It can combine with almost every element to form fluoride.
- It can also react with organic radicals to provide organic fluorides.
Floride Mechanism Of Action
Flouride is anti-cariogenic because it replaces the hydroxyl ion (OH’) in hydroxyapatite with fluoride ion (F) to form flora-petite on the outer surface of the enamel.
Fluorapatite then hardens the enamel and makes it more resistant to acid action. Fluoride also has moderate antibacterial action against cariogenic bacterial flora.
Fluoride Therapy
- Systemic Fluoride
- Fluoridation of drinking water
- Fluoride supplements, i.e. tablets, lozenges, chewing gums.
- Topical Fluoride
- Self-applied topical fluorides
- Fluoride toothpaste and gels
- Fluoride mouth rinses
- Fluoride dentifrices, i.e. sodium floride dentrifrices, stannous floride dentrifrices.
- Topical application of fluorides by a dentist
- Solutions, gels, and foams
- Varnishes.
Higher doses of fluoride lead to fluoride toxicity which is fatal.
Question 20. Write Short Note On Tooth Caries.
Or
Write Short Note On Caries And Floride.
Answer:
Dental Caries And Their Treatment
Dental caries is a slowly progressive, degenerative, infective, irreversible condition characterized by demineralization and decay of hard and soft parts of the teeth resulting in cavitations and disintegration.
- Role of carbohydrates and acids: Easily fermentable carbohydrates, for Example. sucrose plays an important role in the development of caries. Fermentation of carbohydrates in the oral cavity results in the production of acids like lactic acid, aspartic acid, etc. These acids are responsible for the demineralization and decalcification of the teeth. Carbohydrates also lead to the synthesis of certain polysaccharides in the presence of certain enzymes, these polysaccharides hold the plaque tightly over the tooth surface and therefore responsible for caries.
- Role of microorganisms: Dental caries is a bacterial disease. Streptococcal mutants and lactobacilli are the important strains responsible for caries. These bacteria form a large amount of sticky, insoluble polysaccharides and also produce lactic acid from sucrose which is responsible for caries.
- Role of plaque: Plaque is a thin transparent, mucinous film over the tooth surface. This film may hold the bacteria over the tooth surface. It also prevents the escape of acid into saliva and is therefore indirectly responsible for dental caries. Thus both organic and inorganic matter of the teeth are destroyed. As the process continues, the pulp is penetrated and the infection may spread into the systemic circulation.
Tooth Caries Prophylaxis (Prevention Of Caries)
Dental caries can be prevented by the following measures:
- Public education especially to children regarding dental hygiene, proper use of toothbrushes, dentifrices, and prevention of caries (early management)
- Soluble carbohydrates should be avoided especially in the person who is suffering from dental caries. Sweets, ice creams, chocolates, etc. are rich sources of soluble carbohydrates and are also good substrates for bacterial growth.
- “Fluoride therapy” can be used to increase the resistance of the host.
- Prevent the formation of plaque, by using antiplaque drugs.
- Try to reduce ‘in-between’ eating habits.
Tooth Caries Treatment
Fluoride, a halogen has been shown to reduce the incidence of dental caries.
Application Of Fluoride
Systemic Administration
Fluoridation via Water Supply
- 0.5–l ppm, i.e. 1 part of fluorine to 1 million parts of the water is considered adequate for prophylaxis purposes. Fluoridation of drinking water is the most effective measure of permanent teeth. Several large-scale studies have established the beneficial effects of optimum fluoridation of communal water supplies in preventing caries. More than 1–2 ppm results in toxicity, and causes dental fluorosis.
- Adequate prophylaxis can also be achieved by supplementing fluorine in the form of sodium tablets in the diet. It is suggested that using one tablet a day during the period of tooth development for nursing mothers and children up to the completion of calcification of third molars.
Fluoridation via Salt
In Europe, table salt is fortified with 200 to 350 mg/Kg of salt in deficient areas.
Sodium Fluoride Tablets/Lozenges/Drops
Infants and children in areas where there is no mass fluoridation are protected from a high incidence of dental caries by giving sodium fluoride tablets, lozenges, and drops till the age of 16 years. Lozenges and drops also provide a local application to the teeth.
Topical Application
- Fluoridated toothpaste: Common salt added to fluoridated toothpaste is sodium mono fluorophosphate 0.76%. Other salts used have some limitations. Care should be taken to avoid ingestion of fluoridated toothpaste.
- Fluoride mouth rinses: Sodium fluoride or stannous fluoride are used as daily mouth rinses in susceptible individuals to prevent dental caries. The rinse solution is held in the mouth for 1 to 3 min and is swished around. It is now discarded and food/drink are avoided for 30min to minimize washing away of fluoride in contact with teeth.
- Professionally applied fluoride: This is done by the dentist at long intervals.
- Acidulated phosphate fluoride (APF): Formulated as a gel or solution it consists of 1.23% fluoride and 0.1M orthophosphoric acid. Ph is adjusted to 3. Acidic medium enhances fluoride diffusion into the enamel while orthophosphoric acid prevents enamel dissolution. Applications are repeated at 6 months of interval and the optimal duration of each application is 4 min.
- Fluoride varnish: They are non-aqueous preparations that are not washed off by saliva and are retained on teeth for longer periods. A 2% sodium fluoride lacquer in resin base or a polyurethane varnish containing 0.7% fluoride is painted on teeth or applied to the oral cavity.
Adjuvant Anticaries Agents
- Chlorhexidine mouth rinses (0.12%) can also be recommended to a patient with dental caries (in addition to fluorides) as it reduces the levels of Streptococcus mutants, an important member of cariogenic flora. Staining of teeth is associated with the overuse of chlorhexidine.
- Triclosan is a phenolic antimicrobial agent that has been used clinically to treat gingivitis and to prevent plaque, caries, and tartar. It also inhibits the growth of cariogenic Streptococcus mutans. It is not used alone.
- Some multipurpose kinds of toothpaste (anticavity, antiplaque, and antigingivitis) like Colgate Total contain 0.3% triclosan along with 0.24% sodium fluoride incorporated in a polyvinyl copolymer.
Question 21. Explain Why Florides Are Used In Dental Caries.
Answer:
Flouride is used in dental caries because it is anti-cariogenic, i.e. it replaces the hydroxyl ion (OH’) in hydroxyapatite with fluoride ion (F) to form fluorapatite on the outer surface of the enamel.
Fluorapatite then hardens the enamel and makes it more resistant to acid action. Fluoride also has moderate antibacterial action against cariogenic bacterial flora.
Fluoride prevents the decalcification of enamel by acid and prevents the formation of dental caries. Fluoride leads to the remineralization of enamel which gets demineralized.
Fluoride gets concentrated in plaque and inhibits microbial enzymes needed for the production of acid.
Question 22. Briefly Explain The Etiology
Answer:
Etiology Of Dental Caries
“Dental caries is an irreversible progressive microbial disease of the calcified tissues of the teeth, characterized by the demineralization of the inorganic portion and distortion of the organic substances of the tooth, which often leads to cavitation.”
Following is the etiology of dental caries:
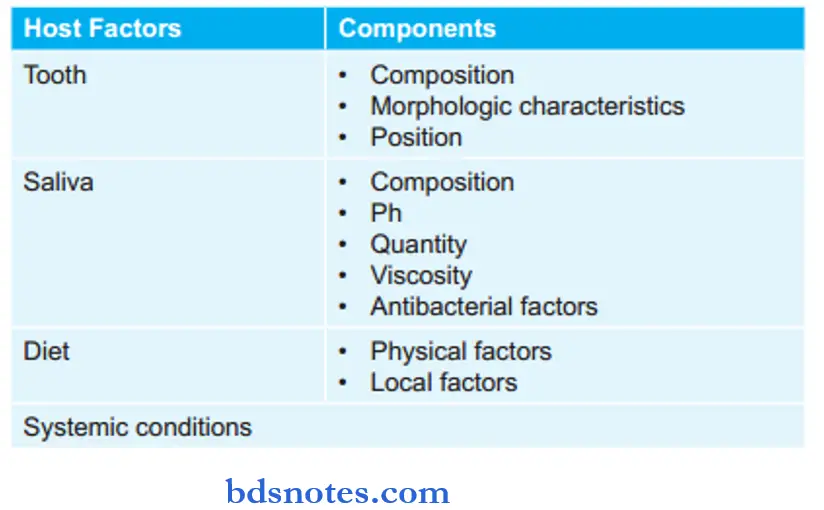
1. Tooth
Composition
- The structure and composition of the teeth influence the initiation and rate of progression of dental caries.
- Surface enamel is more resistant to caries as compared to subsurface enamel. Surface enamel consists of more quantity of fluoride, zinc, lead, and iron. The concentration of carbonate, magnesium, and sodium is lower in the surface layer. The level of carbon dioxide is also lower in the surface layer which causes the dissolution of the surface layer by acids at a lower rate and it consists of less organic and water content.
- Age changes in enamel such as a decrease in the density and permeability and an increase in nitrogen and fluoride content cause teeth to become more caries-resistant.
Morphologic Characteristic
- Deep and narrow occlusal fissures or buccal and lingual pits lead to the development of dental caries.
- As age increases attrition of teeth occurs and this leads to less accumulation of food in fissures, and there is less occurrence of caries.
Position
- A malaligned tooth or the rotated tooth has more chance of predisposition to caries as it tends to accumulate more food debris, cariogenic plaque, and bacteria. In all these teeth cleaning cannot be done.
2. Saliva
Composition
- Inorganic components of saliva: In the normal aspect, saliva is supersaturated with calcium and phosphate ions. This causes the prevention of the dissolution of enamel and also precipitates apatite crystals in the surface of the enamel of carious
lesion which helps in partial repair of tooth damaged by dental caries. During caries, saliva is unsaturated with calcium and phosphate ions which lead to the dissolution of enamel. Fluoride has also got an excellent role in the reduction of dental caries. - Organic components of saliva: High concentration of ammonia retards plaque formation and neutralizes the acid. Urea increases the neutralizing power of saliva. Enzyme salivary amylase leads to the degradation of starch and makes it more soluble in this way starch is washed away from the tooth surface.
pH
- Critical pH is the pH at which saliva appears to be saturated with calcium and phosphorus ions. The value of critical ph is 5.5, below the critical pH inorganic portion of the tooth, starts dissolving. As there is an increase in the concentration of hydrogen ions in cariogenic plaque, this leads to the loss of more phosphate ions from the tooth.
- Buffering property of saliva leads to the diffusion of bicarbonate ions in dental plaque and neutralizes the acid during the caries process.
Quantity
- The quantity of saliva is inversely proportional to dental caries activity.
- More is the salivary flow less is the caries index.
- Hyposalivation occurs due to the conditions like diabetes mellitus, uremia, and usage of antisialogogues.
Viscosity
If saliva is thick mucinous there is a presence of high caries incidence.
Antibacterial Factors
- Saliva consists of many antibacterial products such as lysozyme, salivary peroxidase, and immunoglobulins.
- Lysozyme under the presence of sodium lauryl sulfate can lyse cariogenic streptococci.
- Salivary peroxidase inactivates bacterial enzymes of the glycolytic pathway and inhibits their growth. This is more effective against lactobacillus bacteria.
- IgA immunoglobulin inhibits S. mutans in saliva.
3. Diet
Physical Factors
- Raw unrefined food consists of roughage which cleans the teeth but the presence of soil and sand leads to attrition of occlusal and proximal surfaces of teeth and reduces dental caries.
- Soft and refined foods stick to the teeth and cause increased accumulation of debris which causes an increased risk of dental caries.
Local Factors
- Carbohydrates, i.e. starch, sucrose, lactose, glucose, fructose, or maltose play an important role in the process of dental caries. Synthesis of extracellular polysaccharides, glucans, and levan helps in the adherence of bacteria to teeth.
- In lipids, medium-chain fatty acids and their salts have antibacterial properties at low pH.
- A deficiency of vitamins A and D can lead to enamel hypoplasia which can lead to dental caries in affected teeth.
4. Systemic Conditions
- Hereditary: There is the possibility of dental caries which leads to the inheritance of tooth form or structure which predisposes to dental caries.
- Pregnancy: In the later stages of pregnancy because of a lack of oral hygiene there is an increased risk of dental caries.
Question 23. Write Short Note On Chlorhexidine.
Answer:
Chlorhexidine is an antiplaque agent. Chlorhexidine is a bisbiguanide.
Chlorhexidine Mechanism Of Action
It has a broad spectrum of antibacterial activity:
- Gram-positive bacteria are more susceptible than gram-negative
- In relatively high concentrations it is bacteriocidal but in low concentrations, it may be bacteriostatic
- Cationic molecules of chlorhexidine bind readily to the oppositely charged cell wall and interfere with the membrane transport initiating a leakage of low molecular weight substances
- In high concentrations, chlorhexidine penetrates the cell and causes precipitation of cytoplasm (bactericidal action).
Chlorhexidine Uses
- For wound cleaning
- Disinfecting instruments and storage of instruments
- As preoperative skin scrub
- As disinfectant during root canal treatment
- A daily oral rinse to inhibit the deposition of bacterial plaque on the tooth surface.
Chlorhexidine Adverse Effects
- Locally reversible side effects to chlorhexidine use may occur, primarily brown staining of the teeth, tongue, and silicate and resin restorations.
- Transient impairment of taste perception.
- Painful, desquamative lesions on the oral mucosa may be associated with a burning sensation.
Question 24. Write Short Note On Florides In Dentistry.
Answer:
Fluoride is a halogen and is highly reactive and electronegative.
Florides In Dentistry Mechanism Of Action
- Hydroxyapatite crystals lead to the hardness of tooth enamel. But the hydroxyapatite crystals are readily Fluoride radical is highly reactive, so it exchanges with hydroxyl radical and leads to the formation of fluorapatite. Fluorapatite is more compact, and harder, and is a less acid-labile substance than hydroxyapatite. This leads to teeth becoming more caries-resistant.
- Fluoride also leads to the remineralization of enamel which is attacked by the acid. Free fluoride ion release from fluorapatite by the action of acid raises local fluoride ion concentration and facilitates remineralization of damaged enamel.
- Fluoride inhibits plaque formation and also reduces anaerobic glycolysis and lactic acid formation within the formed plaque.
Application Of Fluoride In Dentistry
A low concentration of fluoride can be applied by the subject himself (daily), or fluoride may be applied at a high concentration by the dentist once a while (generally every 6 months).
- Fluoride toothpaste: This is the easiest and most frequently employed method of fluoride application. Most of the commercially marketed toothpaste now contains fluoride. The most common salt added is sodium monocrotophos- phase at a concentration of 0.76%. Some other compounds and co-polymers have been included in toothpaste to improve the retention of fluoride in the tooth.
- Fluoride mouth rinses: Sodium fluoride (0.055%) and stannous fluoride (0.1%) solutions have been used as daily mouth rinses by susceptible individuals to prevent caries. The rinse solution is held in the mouth for 1–3 min and swished around. It is then discarded and food/drink are avoided for the next 30 min to minimize washing away of fluoride that is in contact with the teeth.
- Though stannous fluoride is more effective than sodium fluoride, it can stain the teeth.
- Professionally applied fluoride: Caries protective effect of fluoride can also be obtained by providing brief intensive exposure to the teeth by the dentist at relatively long intervals.
- Acidulated phosphate fluoride: This fluoride system suitable for application to the teeth by the dentist has been specifically developed to achieve high fluoride permeation into the enamel and thus afford prolonged caries protection. Formulated as a solution or gel, it contains 1.23% fluoride and 0. l M orthophosphoric acid; the pH is adjusted to about 3.0. This acidic medium enhances fluoride diffusion into the enamel while orthophosphoric acid prevents enamel dissolution.
- Fluoride varnishes: These are nonaqueous preparations that are not washed off by saliva and are retained on the teeth for a longer period. A 2% sodium fluoride lacquer in resin base or a polyurethane varnish containing 0.7% fluoride is painted over the teeth or applied to the cavity. The efficacy of these preparations is variable and they are less popular.
Question 25. Define Dentifrices. Describe Dentifrices Under The
Following Headings:
1. Composition
2. Method Of Preparation
3. Method Of Application
Answer:
Dentifrices are the preparations that are used for cleaning the surfaces of teeth and keeping them shiny to preserve the health of teeth and gums.
They are available as tooth powder, toothpaste, gels, dental creams, and even dental foams.
Dentifrices Composition
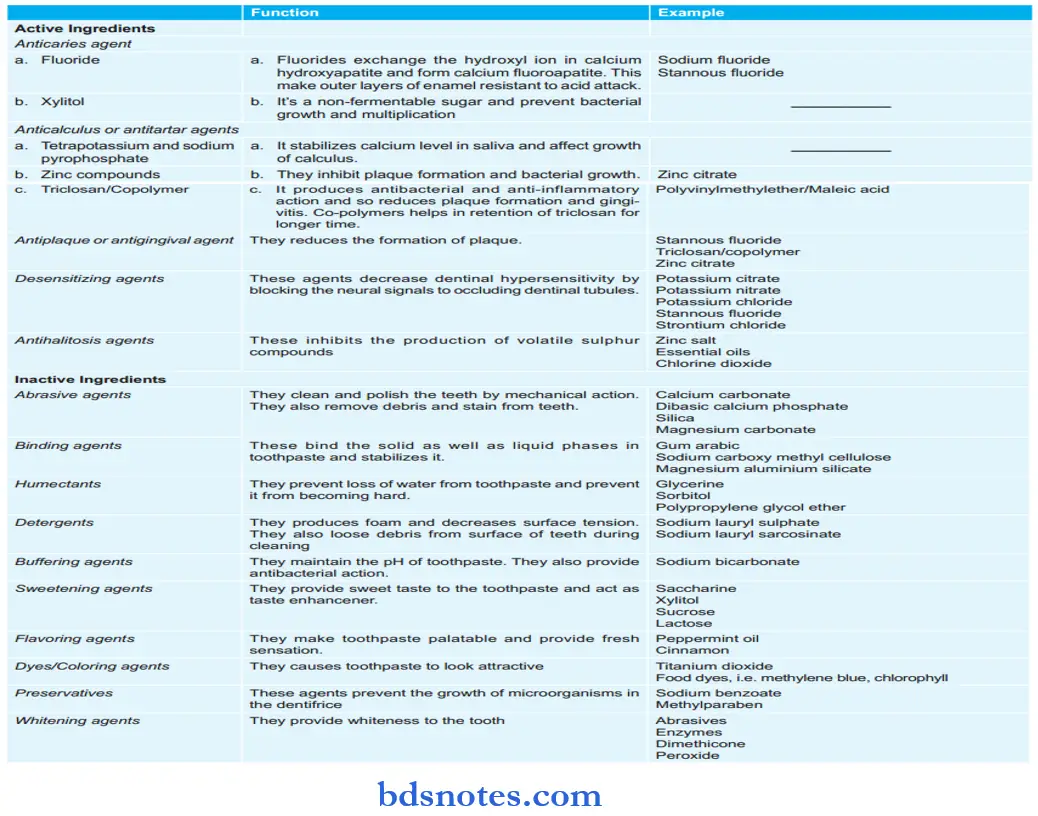
Dentifrices Methods Of Preparation
Preparation Of Toothpaste
Toothpaste is prepared either by the dry gum technique or the wet gum technique.
In the first technique all the solid components, including the binding agent (but excluding surfactants) is first dry mixed, and then the liquid components, that is humectants.
Water is gradually added while in the second technique, the binding agent is first mixed in the liquid phase, a mucilage prepared and then the rest of the solid ingredients are added (except surfactants) and mixed well to produce a homogenous paste mass.
The homogenous paste obtained from either process must then be mixed with both the surfactants and flavored under the vacuum.
There are three methods of preparation of a toothpaste
- Cold method
- Heat liquid phase method
- Multiple liquid phase method.
Cold Method
The humectants such as glycerin or sorbitol are added to the bowl of the mixer. The binder is sprinkled in under agitation so that the particles are dispersed in the absence of water.
To prevent swelling at this point a separate liquid phase is prepared, which includes the available water, sweetener, preservatives, and any therapeutic additives.
This solution is then added to the humectants binder mixer. The mixer is placed under vacuum for 5 min.
To deaerate the thick gelatinous liquid phase the vacuum is open and the abrasives are added with mixing until they are thoroughly wet down.
The vacuum is reapplied and the paste is mixed for at least 30 min under 28 inches or more of vacuum.
In the meantime the surface active agents and flavor are dispersed in about 5% of the available humectants at the conclusion of the 30 min time, the vacuum is again opened, and the flavor mixer is added.
5 min of additional mixing under a vacuum will usually produce a smooth air free paste.
Heat Liquid Phase Process
In this method, the abrasive, binder, and preservatives are premixed as dried powders in the mixer a hot solution of the humectants, water, and sweetener is then slowly added with the mixing of the dried powders.
The resulting mass is mixed under vacuum for 30 min. After which the solution of flavor and surfactant is added for a final 5 min of vacuum mixing
Multiple Liquid Phase Process
This method is particularly adaptable to formulation using a magnesium aluminum silicate-carboxy methyl cellulose binder system.
Magnesium aluminum silicate is added to hot water in the mixing vessels followed by the sweetener a separate phase is prepared consisting of the bulk of the humectants, the binder, the flavor, and the preservatives.
This solution is added to the mixer, followed by the balance of the humectants.
5 min. of vacuum mixing should be performed to desecrate the liquid mixer, abrasives added, and again mixes for 30 min under vacuum after this the surfactant is added in dry form, followed by another 5 min of vacuum mixing.
Preparation Of Toothpowder
The main components of toothpowders are solid particles of very fine size and the end product is also a very dry powder.
Since the main components like abrasives, and surface active agents are solid powders, it is required that they all are in very fine particle sizes, comminuted.
If desired, passed through a sieve and mixed in a mortar on the lab scale and in blenders on an industrial scale.
The flavoring oils are added in the end either by spaying on the powder mixture or first blending with one of the components and then mixing this blend with the rest of the mixture by the method of dilution or geometric progression.
Method Of Application
- Take pea-sized dentifrice over a toothbrush. It should be applied to the toothbrush in such a manner that it completely engages or adhere to the complete length of the bristles of the toothbrush.
- Now brush your teeth with suitable dentifrice and toothbrush for 1 to 2 minutes along with recommended brushing technique.
- After brushing is completed rinse off the mouth thoroughly with water.
Question 26. Describe Mouthwashes Under the Following Headings:
1. Composition
2. Method Of Preparation
3. Method Of Application
Answer:
Mouthwashes are used for rinsing the oral cavity and maintaining oral hygiene.
Mouthwashes Composition
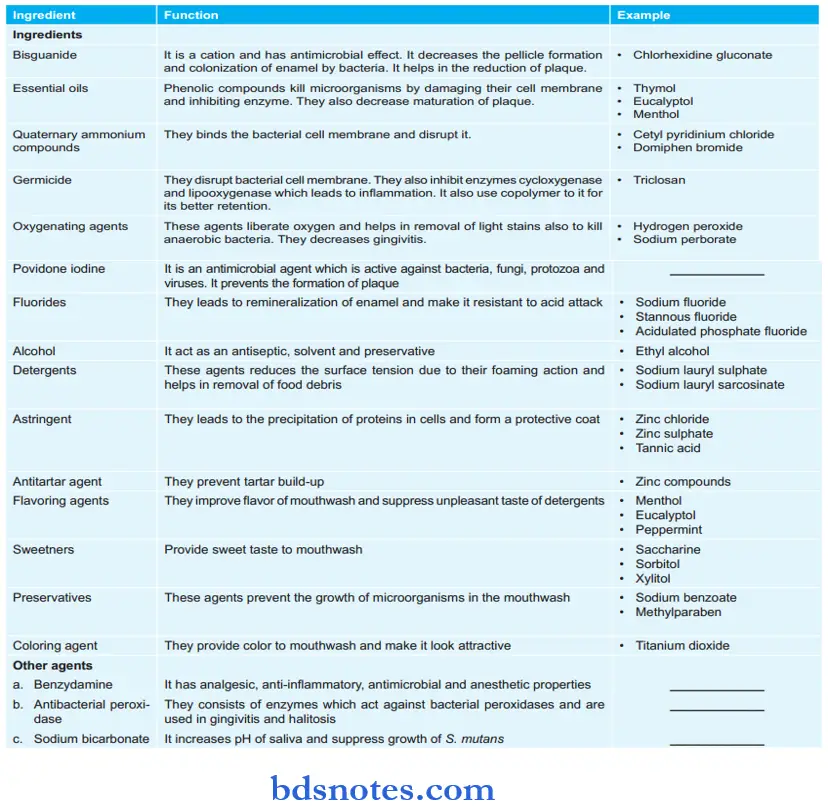
Mouthwashes Methods Of Preparation
The manufacture of mouthwashes is extremely simple and in principle, it only requires one or more stainless steel tanks, an efficient mixture, and a storage tank.
This is because all ingredients are soluble in water and the finished product has a viscosity more or less similar to that of water.
Glass-lined, unpitted mixing tanks are satisfactory for most of the products.
Explosion-proof equipment and a bonded storage area are required, and stringent safety precautions and very strict control of bonded ethanol are maintained.
Mouthwashes Methods Of Application
- The standard amount of mouthwash is enough to clean the teeth in one dose.
- The bottle of mouthwash may have come with a small cup (often the bottle’s cap) one can use to measure the correct amount.
- Tip the cup into the mouth and pour in all of the mouthwash at once.
- Close the mouth to create a seal so that the mouthwash won’t squirt out when one starts swishing it.
- Do not swallow the mouthwash. It may contain strong chemicals that are not meant to be ingested.
- Follow the directions on the bottle to learn exactly how long you should swish the mouthwash.
- Make sure it swishes in front of and behind your teeth. Swish it through your molars as well as your front teeth. Swish it under your tongue and across the roof of your mouth, too.
- When swishing is done, spit it out into the sink.
- Depending on what type of mouthwash you used, one might need to wait 1/2 hour or more before drinking water or eating in order to increase the effectiveness of the mouthwash.
Question 27. Name Dental Desensitizing Agents. Mention Their Mode Of Administration.
Answer:
Name of dental desensitizing agents Following are the dental desensitizing agents:
- Potassium nitrate
- Strontium chloride
- Potassium oxalate
- Fluoride
- Formaldehyde
- Dentin bonding agents.
Dental Desensitizing Agents Mode Of Administration
- Potassium nitrate, strontium chloride, fluoride, and formaldehyde are all included in the desensitizing toothpaste. The paste is to be applied on the sensitive teeth and left in place for 5 min before brushing, then brush lightly and rinse. This is repeated 2 to 3 times a day.
- Fluoride iontophoresis is done by applying electric current via a 2% sodium fluoride solution. This needs special equipment and expertise application. It also needs several repetitions.
- A dentin bonding agent is applied on the tooth by applicator tip or application brush. The sensitive tooth is isolated with the cotton and saliva ejector, the tooth is dried and now a bonding agent is painted to the tooth surface by applicator tip or brush, allowing the bonding agent to dry without coming in contact with saliva.
Question 28. Write A Long Answer On Bleaching Agents Or Whitening Agents.
Answer:
Bleaching agents are the agents which are used to remove stains from teeth or to improve their whiteness.
Most of the bleaching agents act by oxidizing the stain/yellowish coating on the enamel, but a few reducing agents also have stain-removing action.
- Oxygen-releasing agents: These agents release oxygen which reacts with organic pigment to decolorize it and loosen it from the tooth surface. The agent is then washed off to expose the white enamel.
- Hydrogen peroxide is the primary oxygenating agent. Concentrated solution i.e. 20 to 30% in water known as perhydrol or ether named prozone can be applied carefully to the stained teeth and wiped off for cosmetic whitening.
- Burning sensation, erythema, inflammation, and sloughing may occur if it comes in contact with gingival/ oral mucosa.
- Carbamide peroxide is an equimolar complex of hydrogen peroxide with urea which acts as a carrier and releases hydrogen peroxide on reacting with water. Some tooth whiteners consist of 10% carbamide peroxide.
- Sodium peroxide is water soluble and releases oxygen in solution and may be used for bleaching teeth. Sodium perborate is insoluble but slowly releases oxygen on coming in contact with water. It is present in some tooth powders.
- Chlorine-releasing agent: Bleaching powder, i.e. chlorinated lime slowly releases chlorine which acts as an oxidizing agent and decolorizes many of the dyes.
- The addition of acetic acid to bleaching powder immediately before application accelerates its decomposition and hastens stain removal.
- Excessive use of any of the oxidizing bleaching agents can damage the tooth enamel and even affect the dentine. Tooth sensitivity and weakening of the crown may result. The oral microbial flora may also be disturbed.
- Reducing agent: Sodium thiosulfate is a reducing agent which is used for removing certain stains, for Example. iodine stain. Sequential application of an oxidizing agent followed by a reducing agent may be needed for silver stain.
- Silica: It is a nonabrasive adsorbent that is included in some whitening toothpaste and tooth powders. The use of lasers for whitening teeth is increasing in the present era.

Leave a Reply