Drugs Acting On Endocrine System Introduction
The human endocrine system is a group of ductless glands that regulate body processes by secreting chemical substances called hormones. Hormones act on nearby tissues or are carried in the bloodstream to act on specific target organs and distant tissues.
- Diseases of the endocrine system can result from the oversecretion or undersecretion of hormones or from the inability of target organs or tissues to respond to hormones effectively.
- Many different glands make up the endocrine system. The hypothalamus, pituitary gland, and pineal gland are in your brain. The thyroid and parathyroid glands are in your neck.
- The thymus is between your lungs, the adrenals are on top of your kidneys, and the pancreas is behind your stomach. Your ovaries (if you’re a woman) or testes (if you’re a man) are in your pelvic region.
- The steroid is any of a class of natural or synthetic organic compounds characterized by a molecular structure of 17 carbon atoms arranged in four rings. Steroids are important in biology, chemistry, and medicine.
The steroid group includes all the sex hormones, adrenal cortical hormones, bile acids, and sterols of vertebrates, as well as the molting hormones of insects and many other physiologically active substances of animals and plants.
Read and Learn More Medicinal Chemistry II Notes
- Among the synthetic steroids of therapeutic value are a large number of different categories of steroids that are frequently distinguished from each other by names that relate to their biological source For Example., phytosterols (found in plants).
- Adrenal steroids, bile acids, or some important physiological function For Example., progesterones (promoting gestation), androgens (favoring development of masculine characteristics), and cardiotonic steroids (facilitating proper heart function).
- Steroids vary from one another like attached groups, the position of the groups, and the configuration of the steroid nucleus (or gonane). Small modifications in the molecular structures of steroids can produce remarkable differences in their biological activities.
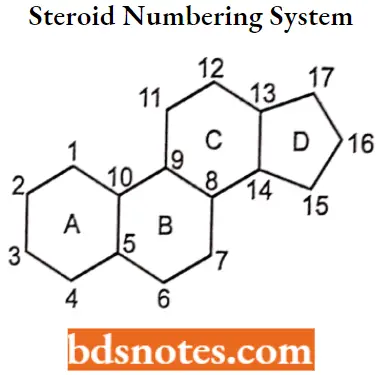
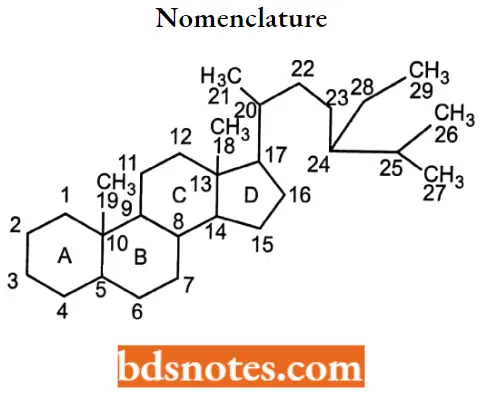
Steroid Numbering System Nomenclature And Stereochemistry
All steroids are related to a characteristic molecular structure composed of 17 carbon atoms arranged in four rings conventionally denoted by the letters A, B, C, and D bonded to 28 hydrogen atoms.
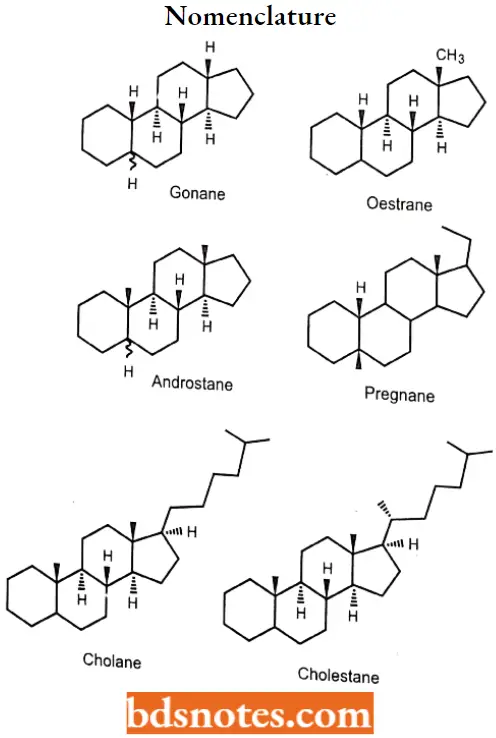
Gonane
This parent structure, named gonane (also known as the steroid nucleus), may be modified in a practically unlimited number of ways by removal, replacement, or addition of a few atoms at a time.
- Hundreds of steroids have been isolated from plants and animals, and thousands more have been prepared by chemical treatment of natural steroids or by synthesis from simpler compounds. The steroid nucleus is a three-dimensional structure, and atoms or groups are attached to it by spatially directed bonds.
- Although many stereoisomers of this nucleus are possible (and maybe synthesized), the saturated nuclear structures of most classes of natural steroids are alike, except at the junction of rings A and B.
- Simplified three-dimensional diagrams may be used to illustrate stereochemical details. For example, androstane, common to several natural and synthetic steroids, exists in two forms (2 and 3), in which the A/B ring fusions are called cis and trans, respectively.
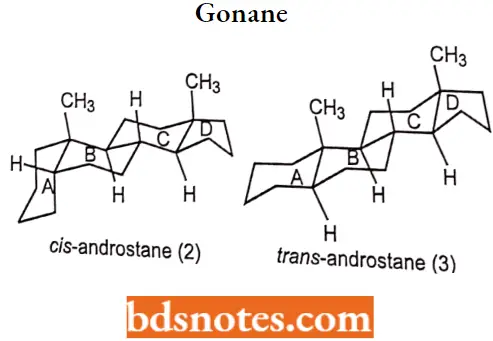
In the cis isomer, bonds to the methyl group, CH3, and the hydrogen atom, H, both project upward from the general plane defined by the rest of the molecule, whereas in the trans isomer.
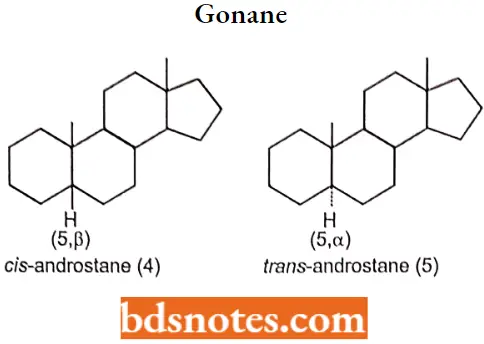
The methyl group projects up and the hydrogen project down. Usually, however, steroid structures are represented as plane projection diagrams such as 4 and 5, which correspond to 2 and 3, respectively.
Stereochemistry of steroids
The stereochemistry of the rings markedly affects the biological activity of a given class of steroids There are six asymmetric carbon atoms 5,8,9,10,13,14 in the nucleus. Therefore 64 optically active forms are possible.
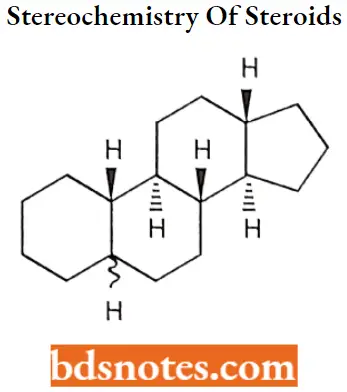
It can exist in two conformations namely chair form and boat form. Chair conformation is more stable than boat conformation due to less angle strain. Hence all cyclohexane rings exist in chair form.
Hydrogen or functional groups on the (3 side of the molecule are denoted by solid lines and the a side is denoted by dotted lines.
Stereoisomerism based on
- How the rings are fused.
- How configuration of substituent groups, particularly those at C3 and C17.
A and B Fusion: Fusion of rings A and B may either trans or cis to give 2 isomeric (alio and normal) C27hydrocarbon.
B and C Fusion: The fusion of the rings B and C was trans. The CIO angular methyl group must be trans to the C9 hydrogen atom.
C and D Fusion: The complete X-ray crystallographic analysis of cholesteryl iodide reveals that the ring union is trans in the sterols, bile acids, and related steroids.
Nomenclature
- Above the plane of the nucleus – β configuration.
- Below the plane of the nucleus – α configuration.
- Configuration of substituents is unknown -wavy line.
- If some carbon atoms are missing in the steroid nucleus, the numbering of the remainder will not change.
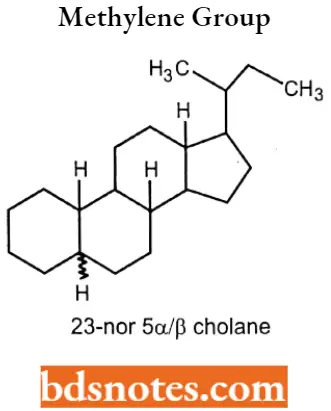
If the side chain doesn’t contain a methylene group, this is indicated by the prefix “nor”, proceeded by the no.of the carbon atom that has disappeared. 23-nor 5α/β cholane.
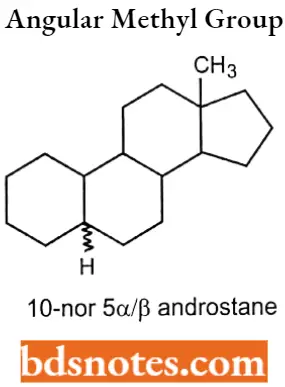
If the steroid nucleus doesn’t contain an angular methyl group, this is indicated by the prefix “nor”, proceeded by the number that designates the methyl group. 10-nor 5α/β androstane.
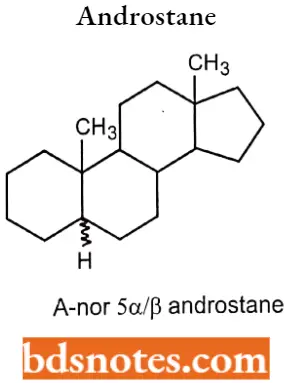
If the ring contraction occurs in the steroid nucleus, this is again indicated by the prefix “nor”, preceded by a capital letter indicating the ring affected. A- nor 5α/β androstane.
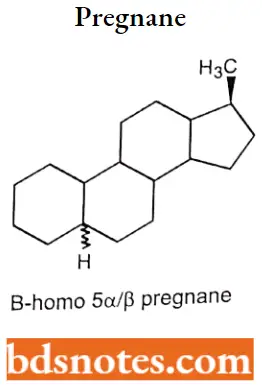
If there is an enlargement of the ring in the steroid nucleus, indicated by the prefix “homo”, proceeded by a capital letter indicating the ring affected. B- homo 5 α/β pregnane.
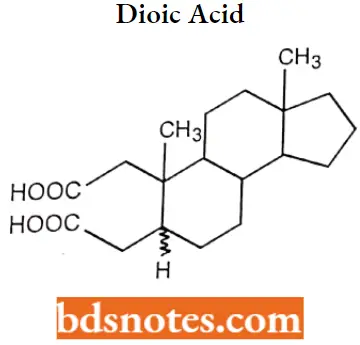
If there is a ring fission or breaking occurs, indicated by the prefix “seco”, proceeded by several positions of a bond broken. 2,3 Seco 5α/β-androstane-2,3-dioic acid.
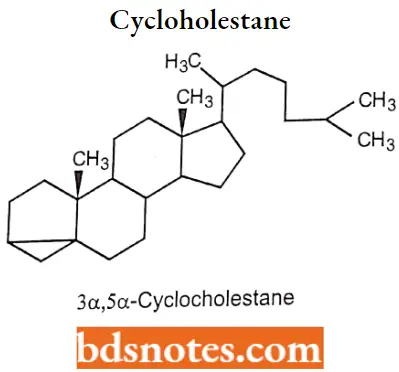
If the steroid nucleus contains 3 membered rings, indicated by the prefix “cyclo”. 3α,5α-Cyclocholestane.
Metabolism of steroids: Steroids are primarily oxidized by cytochrome P450 oxidase enzymes, such as CYP3A4. These reactions introduce oxygen into the steroid ring, allowing the cholesterol to be broken up by other enzymes into bile adds.
- These acids then be eliminated by secretion from the liver in bile. The expression of the oxidase gene can be upregulated by the steroid sensor PXR when there is a high blood concentration of steroids.
- Steroid hormones, lacking the side chain of cholesterol and bile adds., are typically hydroxylated at various ring positions or oxidized at die 17 positions, corrugated with sulfate or glucuronic add, and excreted in the urine.
Sex Hormones
A hormone is a chemical released into the blood and transported to affect cells in other parts of the body. Hormones regulate many things in the body, such as Growth and development.
- Male and female development How tire body uses energy. Levels of salts and sugars in the blood. The amount (volume) of fluid in the body.
- Sex hormone is a chemical substance produced by a sex gland or other organ that affects the sexual features of an organism. Like many other kinds of hormones, sex hormones may also be artificially synthesized.
Types of male and female hormones
Estrogen
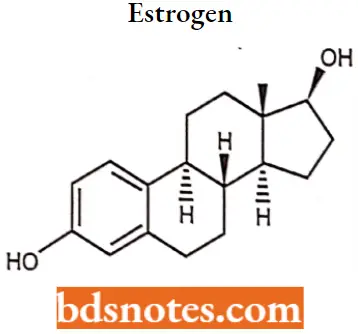
Estrogen IUPAC name: (17β)-estra-1,3,5(10)-triene-3,17-diol.
- Estrogen, or estrogen, is the primary female sex hormone. It is responsible for the development and regulation of the female reproductive system and secondary sex characteristics.
- Three major endogenous estrogens in females have estrogenic hormonal activity: estrone, estradiol, and estriol The estrane steroid estradiol is the most potent and prevalent of these.
- The three major naturally occurring forms of estrogen in females are estrone (El), estradiol (E2), and estriol (E3). Another type of estrogen called estetrol (E4) is produced only during pregnancy.
- Quantitatively, estrogens circulate at lower levels than androgens in both men and women. While estrogen levels are significantly lower in males compared to females, estrogens nevertheless also have important physiological roles in males.
Estrogen MOA: The actions of estrogen are mediated by the estrogen receptor (ER), a dimeric nuclear protein that binds to DNA and controls gene expression.
- Like other steroid hormones, estrogen enters passively into the cell where it binds to and activates the estrogen receptor.
- The estrogen complex binds to specific DNA sequences called a hormone response element to activate the transcription of target genes.
- Since estrogen enters all cells, its actions are dependent on the presence of the ER in the cell. The Er is expressed in specific tissues including the ovary, uterus, and breast.
Estrogen SAR:
- Each estrogen contains a phenolic A ring with a hydroxyl group at carbon 3 and a beta-OH or ketone in position 17 of ring D.
- The phenolic A ring is the principal structural feature responsible for selective, high-affinity binding to both receptors.
- Ring A and 3-OH, 17β-OH groups are necessary for activity.
- Activity depends on the mode of action Subcutaneous= Estradiol > Estrone > Estriol
Oral = Estriol > Estradiol > Estrone. Ethinyl estradiol has the greatest activity, because of resistance to metabolism in the liver and degradation by enzymes in GIT. - Removal of the 3-OH group, epimerization of 17(3-OH group, the introduction of unsaturation in the ‘B’ ring, and expansion of the rD’ ring drastically decrease the activity.
- Compound without steroid nuclei also shows activity.
Estrogen Metabolism: Estrogens are metabolized via hydroxylation by cytochrome P450 enzymes such as CYP1A1 and CYP3A4 and via conjugation by estrogen sulfotransferases (sulfation) and UDPglucuronyltransferases (glucuronidation).
In addition, estradiol is dehydrogenated by 17β- hydroxysteroid dehydrogenase into the much less potent estrogen estrone. These reactions occur primarily in the liver, but also in other tissues.
Estrogen Therapeutic Use: Estrogen is a type of medication that is used most commonly in hormonal birth control and menopausal hormone therapy.
They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens.
Estrogen Adverse Reactions: Side effects of estrogens include breast tenderness, breast enlargement, headache, nausea, fluid retention, and edema among others.
- other side effects of estrogens include an increased risk of blood clots, cardiovascular disease, and when combined with most progestogens, breast cancer.
- In men, estrogens can cause breast development, feminization, infertility, low testosterone levels, and sexual dysfunction among others.
Estradiol
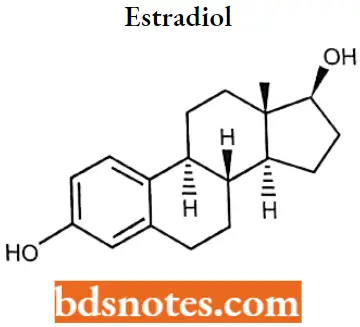
Estradiol IUPAC name: (8R,9S,13S,14S,17S)-13-Methyl 6,7,8,9,ll,12,14,15,16,17decahydrocyclopenta[a]phenanthrene-3,17-diol.
Estradiol MOA: Estradiol acts primarily as an agonist of the estrogen receptor (ER), a nuclear steroid hormone receptor. There are two subtypes of the ER, ERα and ERβ, and estradiol potently binds to and activates both of these receptors.
The result of ER activation is a modulation of gene transcription and expression in ER-expressing cells, which is the predominant mechanism by which estradiol mediates its biological effects in the body.
Estradiol Metabolism: Estradiol is also metabolized via hydroxylation into catechol estrogens. In the liver, it is non-specifically metabolized by CYP1A2, CYP3A4, and CYP2C9 via 2-hydroxylation into 2- hydroxyestradiol.
- And by CYP2C9, CYP2C19, and CYP2C8 via 17p-hydroxy dehydrogenation into estrone, with various other cytochrome P450 (CYP) enzymes and metabolic transformations also being involved.
- Estradiol is additionally conjugated with an ester into lipoidal estradiol forms like estradiol palmitate and estradiol stearate to a certain extent; these esters are stored in adipose tissue and may act as a very long-lasting reservoir of estradiol.
Estradiol Therapeutic uses: Estradiol is used as a medication, primarily in hormone therapy for menopausal symptoms as well as transgender hormone replacement therapy.
Oestriol
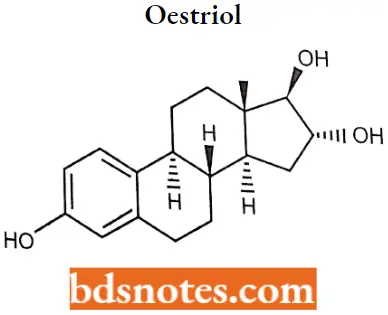
Oestriol IUPAC name: (8R,9S,13S,US,16R,17R)-13-methyl-6<7,8,9,11,12,14,15,16,17-decahydrocycI0penta [a]phenanthrene-3,16,17-triol.
Oestriol MOA: Estriol is an estrogen, specifically an agonist of the estrogen receptors ERα and ERβ. It is a far less potent estrogen than estradiol, and as such is a relatively weak estrogen.
Oestriol Metabolism: Estriol is metabolized via glucuronidation and sulfation.
Oestriol Therapeutic uses: Estriol is used as a medication, primarily in hormone therapy for menopausal symptoms.
Oestriol Adverse reaction: Post-menopausal estrogen therapy is carcinogenic to humans.
Oestrione
Estrone (El), also spelled oestrone, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estriol. Estrone, as well as the other estrogens, are synthesized from cholesterol and secreted mainly from the gonads.
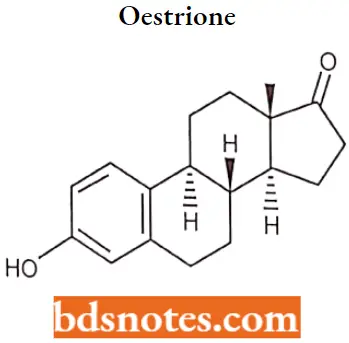
Oestrione IUPAC name: (8R,9S,13S,14S)-3-hydroxy-lS-methyl-ÿ,9,11,12,14,15,16-octahydro-6Hcyclopenta[a]phenanthren-17-one.
Oestrione MOA: Estrone is an estrogen, specifically an agonist of the estrogen receptors ERα and ERβ. it is a far less potent estrogen than estradiol, and as such, is a relatively weak estrogen.
Oestrione Metabolism: Estroen is conjugated into estrogen conjugates such as estrone sulfate and estrone glucuronide by sulfotransferases and glucuronidase, and can also be hydroxylated by cytochrome P450 enzymes into catechol estrogens such as 2-hydroxyestrone and 4-hydroxyestrone or into estriol. Both of these transformations take place predominantly in the liver.
Oestrione Therapeutic uses: Estrone has been available as an injected estrogen for medical use, for instance in hormone therapy for menopausal symptoms, but it is now mostly no longer marketed.
Oestrione Adverse reactions: Bloating, breast tenderness or swelling, swelling in other parts of the body, nausea, leg cramps.
Diethyl stilbestrol
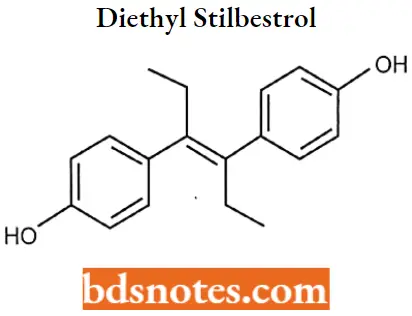
Diethyl stilbestrol IUPAC name: 4-[(E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol.
Diethyl stilbestrol MOA: Estrogens diffuse into their target cells and interact with a protein receptor, the estrogen receptor. Target cells include the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary.
- The effect of Estrogen binding their receptors causes downstream increases in the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) from the anterior pituitary.
- The combination of estrogen with progestin suppresses the hypothalamic-pituitary system, decreasing the secretion of gonadotropin-releasing hormone (GnRH).
Diethyl stilbestrol Metabolism: DES is metabolized mainly by glucuronidation and oxidation, with the latter including aromatic hydroxylation of the ethyl side chains and dehydrogenation into (Z, Z)-dienestrol. It is also known to produce paroxypropione as a metabolite.
Diethyl stilbestrol Therapeutic Uses: DES has been used in the past for the following indications: Recurrent miscarriage in pregnancy Menopausal hormone therapy for the treatment of menopausal symptoms such as hot flashes and vaginal atrophy.
- Hormone therapy for hypoestrogenism (For Example., gonadal dysgenesis, premature ovarian failure, and after oophorectomy). Postpartum lactation suppression to prevent or reverse breast engorgement.
- Gonorrheal vaginitis (discontinued following the introduction of the antibiotic penicillin). Prostate cancer and breast cancer.
Diethyl stilbestrol Adverse Reaction: DES is associated with high rates of side effects including nausea, vomiting, abdominal discomfort, headache, and bloating, with an incidence of 15 to 50%.
Progesterone
Progesterone is considered the counterpart to estrogen. It is the antagonizer to estrogen-driven growth in the lining of the uterus. Progesterone is essential to the premenstrual cycle. It rises during the second part of the cycle to reduce premenstrual syndrome and prepares the uterus for the implantation of a fertilized egg. Additionally, progesterone is needed to support a healthy pregnancy, as low levels can result in a miscarriage.
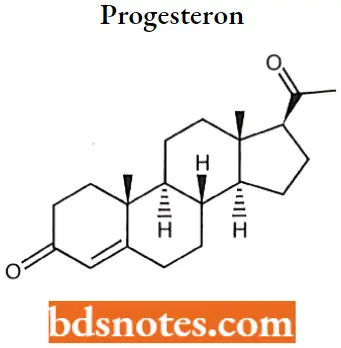
Progesteron IUPAC name: (8S,9S,10R,13S,14S,17S)-17-acetyl-10,13-dimethyl-l,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one.
Progesteron MOA: Progesterone shares the pharmacological actions of the progestins. Progesterone binds to the progesterone and estrogen receptors. Target cells include the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary.
- Once bound to the receptor, progestins like Progesterone will slow the frequency of release of gonadotropin-releasing hormone (GnRH) from the hypothalamus and blunt the pre-ovulatory LH (luteinizing hormone) surge.
- In women who have adequate endogenous estrogen, progesterone transforms a proliferative endometrium into a secretory one. Progesterone is essential for the development of decidual tissue and is necessary to increase endometrial receptivity for the implantation of an embryo.
- Once an embryo has been implanted, progesterone acts to maintain the pregnancy. Progesterone also stimulates the growth of mammary alveolar tissue and relaxes uterine smooth muscle. It has little estrogenic and androgenic activity.
Progesteron SAR: Steroidal nucleus is essential for activity.
- Substitution at 17 α with ethynyl, methyl, and ethyl reduces activity.
- Ethindrone is more active orally than progesterone.
- Removal of CH3 at C19 is more active. For Example. (Norethindrone)
- Unsaturation of the B or C ring of 19-Androstane derivates increases activity.
- Acetylation of the 17β-OH of norethindrone long duration of action.
Progesterone Metabolism: Progesterone is metabolized primarily by the liver largely to pregnanediol and pregnanolones.
Progesteron Therapeutic Uses: Taking progesterone by mouth, and applying progesterone gel into the vagina are effective strategies for treating the absence of menstrual periods in premenopausal women.
Hormone replacement therapy (HKD. Intravaginal progesterone gel (Crinone 8%) is FDA-approved for use as a part of infertility treatment in women.
Progesterone Adverse reactions: Progesterone can cause many side effects including stomach upset, changes in appetite, weight gain, fluid retention, swelling (edema), fatigue, acne, drowsiness or insomnia, allergic skin rashes, hives, fever, headache, depression, breast discomfort or enlargement, premenstrual syndrome (PMS)-like symptoms.
Nandrolone
Nandrolone is an anabolic steroid (a muscle-building chemical) that occurs naturally in the human body, but only in tiny quantities.
It is very similar in structure to the male hormone testosterone and has many of die same effects in terms of increasing muscle mass, without some of the more unwanted side effects.
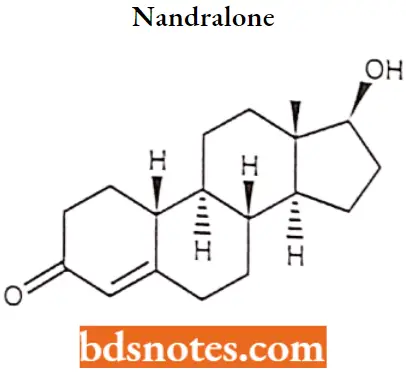
Nandralone IUPAC name: (8R,9S,10R,13S,14S,17S)-17-hydroxy-13-methyl-2,6,7,8,9,10,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-one.
Nandralone MOA: Nandrolone is an androgen receptor agonist. The drug is bound to the receptor complexes which allows it to enter the nucleus and bind directly to specific nucleotide sequences of the chromosomal DNA.
The areas of binding are called hormone response (HREs), and influence transcriptional activity of certain genes, producing the androgen effects.
Nandralone SAR:
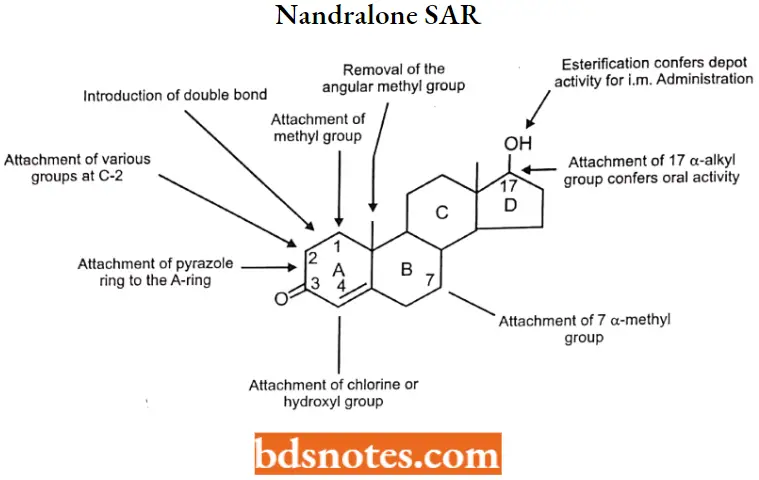
- Some of the structural modifications have been introduced into testosterone in an
attempt to maximize the anabolic effect and minimize the androgenic. - Structural modifications to the A- and B-rings of testosterone increase anabolic activity.
- Substitution at C-17 confers oral or depot activity (i.m.).
Nandralone Metabolism:
Nandrolone is unusual in that unlike most anabolic steroids, it is not broken down into the more reactive DHT by the enzyme 5a-reductase, but rather into a less effective product known as Dihydronandrolone.
Nandrolone Therapeutic Use: Nandrolone esters are used clinically, although increasingly rarely, for people in catabolic states with major bums, cancer, and AIDS, and an ophthalmological formulation was available to support cornea healing.
- The positive effects of nandrolone esters include muscle growth, appetite stimulation increased red blood cell production,[medical citation needed], and bone density.
- Clinical studies have shown them to be effective in treating anemia, osteoporosis, and breast cancer. Nandrolone sulfate has been used in an eye drop formulation as an ophthalmic medication.
Nandrolone Adverse reactions: Side effects of nandrolone esters include masculinization among others. Other side effects of high doses of nandrolone can include erectile dysfunction and cardiovascular damage.
As well as several ailments resulting from the drug’s effect of lowering levels of luteinizing hormone through negative feedback.
Testosterone
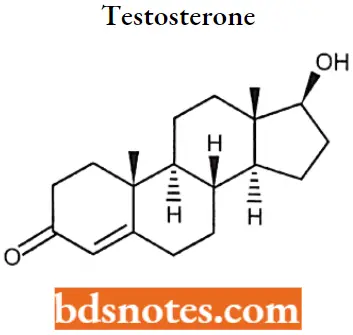
Testosterone IUPAC name: (8R,9S,10R,13S,14S,17S)-17-hydroxy-13-methyl-2,6,7,8,9,10,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-one.
Testosterone MOA: The effects of testosterone in humans and other vertebrates occur by way of two main mechanisms: by activation of the androgen receptor (directly or as DHT), and by conversion to estradiol and activation of certain estrogen receptors.
- Free testosterone (T) is transported into the cytoplasm of target tissue cells, where it can bind to the androgen receptor, or can be reduced to 5a-dihydrotestosterone (DHT) by the cytoplasmic enzyme 5a-reductase.
- DHT binds to the same androgen receptor even more strongly than T, so that its androgenic potency is about 2.5 times that of T. The T-receptor or DHT-receptor complex undergoes a structural change that allows it to move into the cell nucleus and bind directly to specific nucleotide sequences of the chromosomal DNA.
- The areas of binding are called hormone response elements (HREs), and influence transcriptional activity of certain genes, producing the androgen effects.
Testosterone SAR:
- It lacks the 2-carbon side chain attached to the 17 position, making it a 19-carbon steroid (an androstane).
- 17 alpha methyl group increases the duration of action and improves bioavailability. But this change increases hepatotoxicity.
- The esterification of 17 in the beta OH group increases the duration of action and bioavailability.
- The removal of 19th carbon led to more anabolic selective molecules and less androgenic selectivity.
Testosterone Metabolism: Testosterone is metabolized to 17-keto steroids through two different pathways. The major active metabolites are estradiol and dihydrotestosterone (DHT).
Testosterone Therapeutic Uses:
- Use in males: For management of congenital or acquired hypogonadism, hypogonadism associated with HIV infection, and male climacteric (andropause).
- Use in females: For the palliative treatment of androgen-responsive, advanced, inoperable, metastatic (skeletal) carcinoma of the breast in women who are 1-5 years postmenopausal.
- Testosterone esters may be used in combination with estrogens in the management of moderate to severe vasomotor symptoms associated with menopause in women who do not respond adequately to estrogen therapy alone.
Testosterone Adverse Reactions: Common side effects from testosterone medication include acne, swelling, and breast enlargement in males. Serious side effects may include liver toxicity, heart disease, and behavioral changes.
Women and children who are exposed may develop virilization. It is recommended that individuals with prostate cancer not use the medication. lt can cause harm if used during pregnancy or breastfeeding.
Drugs For Erectile Dysfunction
Erectile dysfunction is the consistent or recurrent inability of a man to attain and/or maintain an erection sufficient for sexual performance. ED happens when blood doesn’t flow well to the penis, or when the nerves in the penis are harmed.
ED can signal an underlying health or emotional problem. Finding and treating the cause(s) of your ED can improve your overall health and your sex life.
Sildenafil
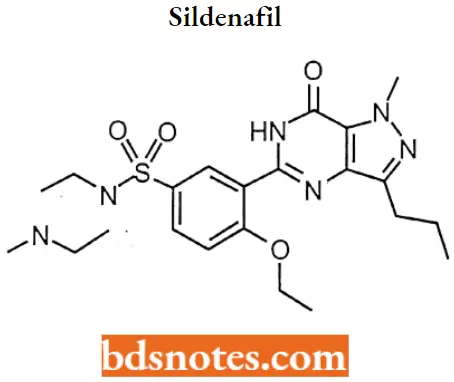
Sildenafil IUPAC Name: 5-{2-Ethoxy-5-[(4-methylpiperazin-l-yl)sulfonyl]phenyl}-1-methyl-3-propyl-1,6- dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one.
Sildenafil MOA: Sildenafil inhibits the cGMP-specific phosphodiesterase type 5 (PDE5) which is responsible for the degradation of cGMP in the corpus cavernosum located around the penis.
- Penile erection during sexual stimulation is caused by increased penile blood flow resulting from the relaxation of penile arteries and corpus cavernosal smooth muscle.
- This response is mediated by the release of nitric oxide (NO) from nerve terminals and endothelial cells, which stimulates the synthesis of cGMP in smooth muscle cells.
- Cyclic GMP causes smooth muscle relaxation and increases blood flow into the corpus cavernosum. The inhibition of phosphodiesterase type 5 (PDE5) by sildenafil enhances erectile function by increasing the amount of cGMP.
Sildenafil Metabolism: Sildenafil appears to be completely metabolized in the liver to 16 metabolites. Its metabolism is mediated mainly by cytochrome P450 microsomal isozymes 3A4 (major route) and 2C9 (minor route).
- The major circulating metabolite, N-demethylated metabolite, has PDE selectivity similar to the parent drug and ∼50% of its in vitro potency.
- The N-demethylated metabolite is further metabolized to an N-dealkylated N, N-de-ethylated metabolite. Sildenafil also undergoes N-dealkylation followed by N-demethylation of the piperazine ring.
Sildenafil Therapeutic Uses: The primary indication of sildenafil is the treatment of erectile dysfunction (inability to sustain a satisfactory erection to complete intercourse).
Its use is now one of the standard treatments for erectile dysfunction, including for men with diabetes mellitus. Sildenafil and other PDE5 inhibitors are used off-label to alleviate vasospasm and treat severe ischemia and ulcers in fingers and toes for people with secondary Raynaud’s phenomenon.
Sildenafil Adverse reactions: Adverse effects of sildenafil use included headache, flushing, indigestion, nasal congestion, and impaired vision, including photophobia and blurred vision.
Some sildenafil users have complained of seeing everything tinted blue (cyanopsia). Some complained of blurriness and loss of peripheral vision.
Tadalafil
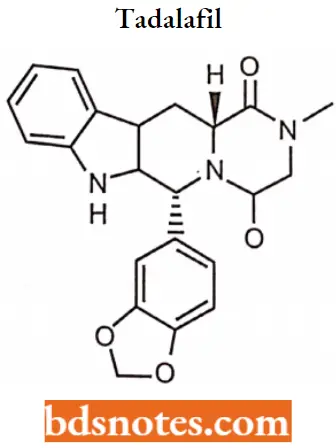
Tadalafil IUPAC name: (6R,12aR)-6-(1,3-benzodioxol-5-yl)-2-methyl-2,3,6,7,12,12a hexahydropyrazino [1′,2′:1,6] pyrido[3,4-b]indole-1,4-dione.
Tadalafil MOA: Tadalafil inhibits the cGMP-specific phosphodiesterase type 5 (PDE5) which is responsible for the degradation of cGMP in the corpus callosum located around the penis.
- Penile erection during sexual stimulation is caused by increased penile blood flow resulting from the relaxation of penile arteries and corpus cavernosal smooth muscle.
- This response is mediated by the release of nitric oxide (NO) from nerve terminals and endothelial cells, which stimulates the synthesis of cGMP in smooth muscle cells.
- Cyclic GMP causes smooth muscle relaxation and enhances the corpus callosum. The inhibition of phosphodiesterase-type erectile function by increasing the amount of cGMP.
Tadalafil Metabolism: Tadalafil is predominantly metabolized by CYP3A4 to a catechol metabolite. The catechol metabolite undergoes extensive methylation and glucuronidation
to form the methyl catechol and methyl catechol glucuronide conjugate, respectively.
In vitro data suggests e metabolites are not expected to be pharmacologically active at observed metabolite concentrations.
Tadalafil Therapeutic Uses: Tadalafil is used to treat male sexual function problems (impotence or erectile dysfunction ED). In combination with sexual stimulation, tadalafil works by increasing blood flow to the perns to help a man get and keep an erection.
- Tadalafil is also used to treat the symptoms of an enlarged prostate (benign prostatic hyperplasia-BPH).
- It helps to relieve symptoms of BPH such as difficulty in beginning the flow of urine, weak stream, and the need to urinate frequently or urgently (including during the middle of the night). Tadalafil is thought to work by relaxing the smooth muscle in the prostate and bladder.
Tadalafil Aderse Reactions: The most common side effects when using tadalafil are headache, stomach discomfort or pain, indigestion, burping, add reflux, back pain, muscle aches, flushing, and stuffy or runny nose.
- These side effects reflect the ability of PDE5 inhibition to cause vasodilation (causing blood vessels to widen) and usually go away after a few hours.
- Back pain and muscle aches can occur 12 to 24 hours after taking the drug, and the symptom usually disappears after 48 hours.
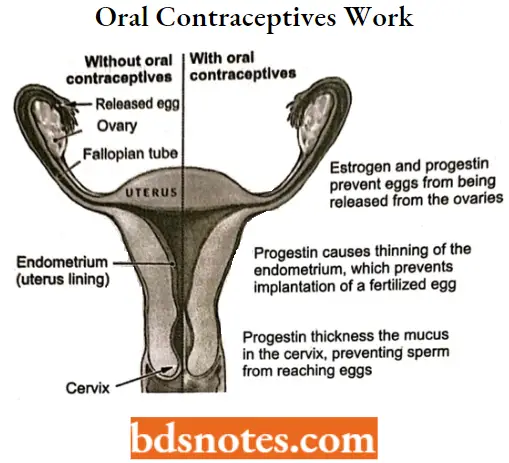
Oral Contraceptives
Oral contraceptives, also called birth control pills, are a safe and reliable option for preventing unwanted pregnancy. Most oral contraceptives contain a combination of 2 types of hormones estrogen and progestin.
- Both of these hormones are naturally found in women’s bodies. There are many different types of estrogen and progestin.
- Both of these hormones are naturally found in women’s bodies. There are many different types of estrogens, and different types of pills contain different combinations, but they all work similarly. some pills contain only progestin, sometimes called the “mini-pill”.
Mifepristone
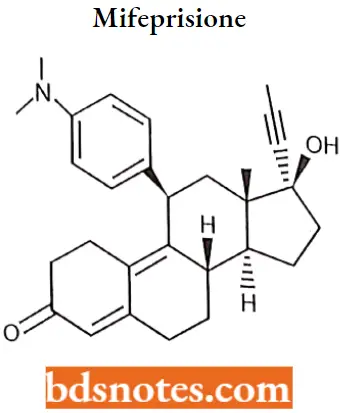
Mifepristone IUPAC name: (8S,11R,13S,14S,17S)-11-[4-(dimethylamino)phenyl]-17-hydroxy-13-methyl-17-prop-1-ynyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenanthren-3-one.
Mifepristone MOA: The anti-progestational activity of mifepristone results from competitive interaction with progesterone at progesterone-receptor sites.
Based on studies with various oral does in several animal species (mouse, rat, rabbit, and monkey), the compound inhibits the activity of endogenous or exogenous progesterone. The termination of pregnancy results.
Mifepristone Metabolism: Hepatic, by Cytochrome P450 3A4 isoenzyme to the N-monomethylated metabolite (RU 42 633); RU 42 698, which results from the loss of two methyl groups from position 11 beta; and RU 42 698, which results from terminal hydroxylation of the 17-propynyl chain.
Mifepristone Therapeutic Uses: Mifepristone followed by a prostaglandin analog (misoprostol or gemeprost) is used for medical abortion. Medical organizations have found this combination to be safe and effective.
Mifepristone is used for the medical treatment of high blood sugar (hyperglycemia) caused by high cortisol levels in the blood (hypercortisolism) in adults with endogenous Cushing’s syndrome who have type 2 diabetes mellitus or glucose intolerance and have failed surgery or cannot have surgery. Mifepristone is used for emergency contraception.
Mifepristone Adverse Reactions: Nausea or vomiting, decreased appetite, dry mouth, tiredness, dizziness, headache, or joint or muscle pain may occur.
Norgestril
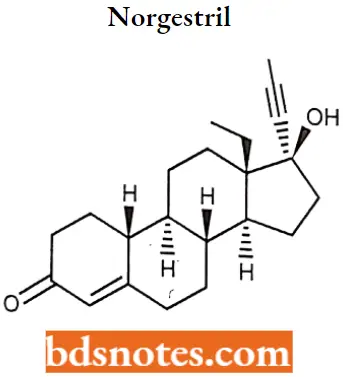
Norgestril IUPAc name: (8R,9S,10R,13S,14S)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-one.
Norgestril MOA: Norgestrel (and more specifically the active stereoisomer levonorgestrel) binds to the progesterone and estrogen receptors with the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary.
Once bound to the receptors, progestins like levonorgestrel will slow the frequency of release of gonadotropin-releasing hormone (GnRH) from the hypothalamus and blunt the pre-ovulatory LH (luteinizing hormone) surge. Loss of the LH surge inhibits ovulation and thereby prevents pregnancy.
Norgestrel Therapeutic Uses: Norgestrel is used in combination with ethinylestradiol or quinestrol in combined birth control pills, alone in progestogen-only birth control pills, and in combination with estradiol or conjugated estrogens in menopausal hormone therapy.
Norgestril Adverse Reactions: Side effects of norgestrel include menstrual irregularities, headaches, nausea, breast tenderness, mood changes, acne, increased hair growth, and others.
Levonorgestrol
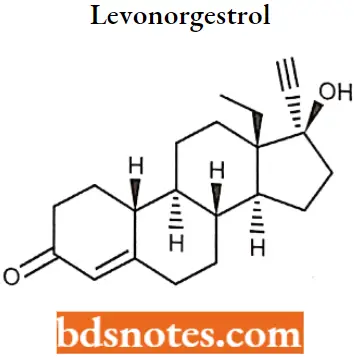
Levonorgestrol IUPAC name: (8R,9S,10R,13S,14S)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,ll, 12,14,15, 16-dodecahydrocyclopenta[a]phenanthren-3-one.
Levonorgestrol MOA: Binds to the progesterone and estrogen receptors. Target cells include the female reproductive tract, the mammary gland, the hypothalamus, and the pituitary.
Once bound to the receptor, progestins like levonorgestrel will slow the frequency of release of gonadotropin-releasing hormone (GnRH) from the hypothalamus and blunt the pre-ovulatory LH (luteinizing hormone) surge.
Levonorgestrol Therapeutic Uses: Levonorgestrel is a hormonal medication that is used in several birth control methods. In pill form, sold under the brand name Plan B among others, it is useful within 120 hours as emergency birth control.
- It becomes less effective the longer after sex and only works before pregnancy has occurred. It is also combined with estrogen to make combined oral birth control pills.
- Within an intrauterine device (IUD), sold as Mirena among others, it is effective for long-term prevention of pregnancy.
Levonorgestrel Adverse Reaction: Common side effects include nausea, breast tenderness, headaches, and increased, decreased, or irregular menstrual bleeding.
Corticosteroids
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogs of these hormones.
- Two main classes of corticosteroids, glucocorticoids, and mineralocorticoids, are involved in a wide range of physiological processes, including stress response, and immune response.
- And regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels, and behavior.
- Glucocorticoids (anti-inflammatory) which suppress inflammation and immunity and assist in the breakdown of fats, carbohydrates, and mineralocorticoids (salt retaining) that regulate the balance of salt and water in the body.
Cortisone
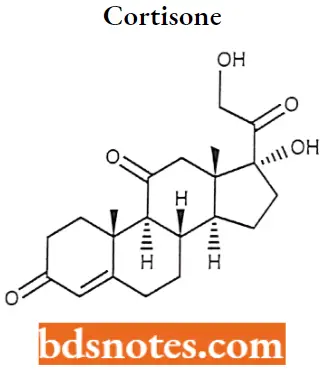
Cortisone IUPAC Name: (8S,9S,10R,13S,14S,17R)-17-Hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8, 9,12,14,15,16-decahydrocydopenta[a]phenanthrene-3,11-dione.
Cortisone MOA: Cortisone suppresses the immune system, thus reducing inflammation and attendant pain and swelling at the site of the injury.
Cortisone Therapeutic Uses: Cortisone, a glucocorticoid, and adrenaline are the main hormones released by the body as a reaction to stress. They elevate blood pressure and prepare the body for a fight-or-flight response.
- A cortisone injection can also be used to give short-term pain relief and reduce the swelling from inflammation of a joint, tendon, or bursa in, for example, the joints of the knee, or elbow, and response in person with autoimmune diseases or following an organ transplant to prevent transplant rejection. [citation needed]
- The suppression of the immune system may also be important in the treatment of inflammatory conditions.
Cortisone Adverse Reactions: Oral use of cortisone has several potential systemic side-effects: Asthma, hyperglycemia, insulin resistance, diabetes mellitus, osteoporosis, anxiety, depression, amenorrhoea, cataracts, Cushing’s syndrome, and glaucoma, among other problems.
Local side effects are rare but can include: pain, infection, skin pigment changes, loss of fatty tissue, and tendon rupture.
Hydrocortisone
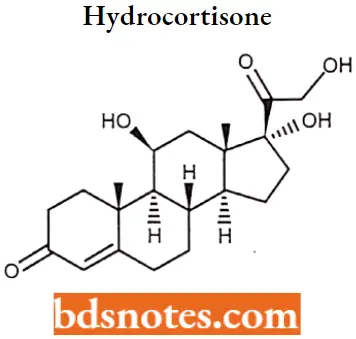
Hydrocortisone IUPAC name: (11β)-11,17,21-trihydroxypregn-4-ene-3,20-dione.
Hydrocortisone MOA: Hydrocortisone binds to the cytosolic glucocorticoid receptor. After binding the receptor the newly formed receptor-ligand complex translocates itself into the cell nucleus, where it binds to many glucocorticoid response elements (GRE) in the promoter region of the target genes.
- The DNA-bound receptor then interacts with basic transcription factors, causing an increase in the expression of specific target genes.
- The anti-inflammatory actions of corticosteroids are thought to involve lipocortins and phospholipase A2 inhibitory proteins which, through inhibition of arachidonic acid, control the biosynthesis of prostaglandins and leukotrienes.
Hydrocortisone Metabolism: Metabolism primarily hepatic via CYP3A4.
Hydrocortisone Therapeutic Uses: Uses include conditions such as adrenocortical insufficiency, adrenogenital syndrome, high blood calcium, thyroiditis, rheumatoid arthritis, dermatitis, asthma, and COPD.
It is the treatment of choice for adrenocortical insufficiency. It can be given by mouth, topically, or by injection. Stopping treatment after long-term use should be done slowly.
Hydrocortisone Adverse reactions: Side effects may include mood changes, increased risk of infection, and swelling. With long-term use common side effects include osteoporosis, upset stomach, physical weakness, easy bruising, and yeast infections. While used, it is unclear if it is safe during pregnancy.
Prednisolone
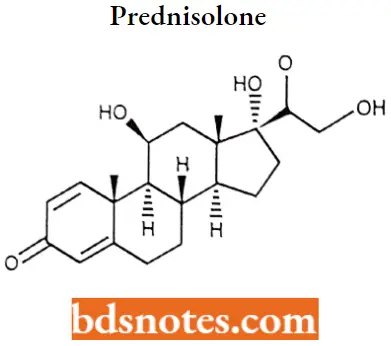
Prednisolone IUPAC name: (11(β)-11,17,21-Trihydroxypregna-1,4-diene-3,20-dione.
Prednisolone MOA: Glucocorticoids such as Prednisolone can inhibit leukocyte infiltration at the site of inflammation interfere with mediators of inflammatory response, and suppress humoral immune responses.
- The antiinflammatory actions of glucocorticoids are thought to involve phospholipase A2 inhibitory proteins, and lipocortins, which control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes.
- Prednisolone reduces inflammatory reactions by limiting the capillary dilatation and permeability of the vascular structures.
Prednisolone Metabolism: Excreted in the urine as either free or glucoconjugate.
Prednisolone Therapeutic Uses: useful for the treatment of a wide range of inflammatory and autoimmune conditions such as asthma, uveitis, pyoderma gangrenosum, rheumatoid arthritis, urticaria, angioedema, ulcerative colitis.
Pericarditis, temporal arteritis, Crohn’s disease, Bell’s palsy, multiple sclerosis, cluster headaches, vasculitis, acute lymphoblastic leukemia and autoimmune hepatitis, systemic lupus erythematosus, Kawasaki disease dermatomyositis, sarcoidosis.
Prednisolone Adverse Reactions: Increased appetite, weight gain, nausea and malaise. Increased risk of infection. Cardiovascular events in children.
- Dermatological effects include reddening of the face, brushing or skin discoloration, impaired wound healing, thinning of the skin, skin rash, fluid buildup, and abnormal hair growth. Hyperglycemia; patients with diabetes may need increased insulin or diabetic therapies for menstrual abnormalities.
- Less response to hormones, especially during stressful instances such as surgery or illness. change in electrolytes: rise in blood pressure.
Betamethasone
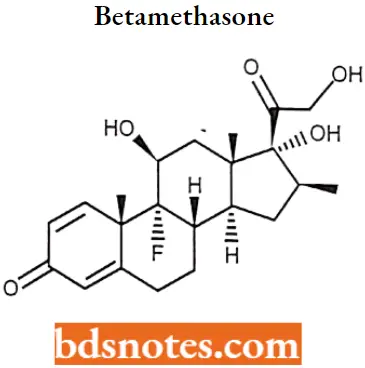
Betamethasone IUPAC name: (8S,9R,10S,11S,13S,14S,16S,17R)-9-Fluoro- 11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl- 6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro- 3H-cyclopenta[a]phenanthren-3-one.
Betamethasone MOA: Betamethasone is a glucocorticoid receptor agonist. This leads to changes in genetic expression once this complex binds to the GRE.
- The antiinflammatory actions of corticosteroids are thought to involve lipocortins and phospholipase A2 inhibitory proteins which, through inhibition of arachidonic acid, control the biosynthesis of prostaglandins and leukotrienes.
- The immune system is suppressed by corticosteroids due to a decrease in the function of the lymphatic system, a reduction in immunoglobulin and complement concentrations, the precipitation of lymphocytopenia, and interference with antigen-antibody binding.
- Betamethasone binds to plasma transcortin, and it becomes active when it is not bound to transcortin.
Betamethasone Therapeutic Uses: It is used for several diseases including rheumatic disorders such as rheumatoid arthritis and systemic lupus erythematosus, skin diseases such as dermatitis and psoriasis, allergic conditions such as asthma and angioedema.
Preterm labor to speed the development of the baby’s lungs, Crohn’s disease, cancers such as leukemia, and fludrocortisone for adrenocortical insufficiency.
Betamethasone Adverse reactions: Serious side effects include an increased risk of infection, muscle weakness, severe allergic reactions, and psychosis. Long-term use may cause adrenal insufficiency.
Stopping the medication suddenly following long-term use may be dangerous. The cream commonly results in increased hair growth and skin irritation. Betamethasone belongs to the glucocorticoid class of medication.
Dexamethasone
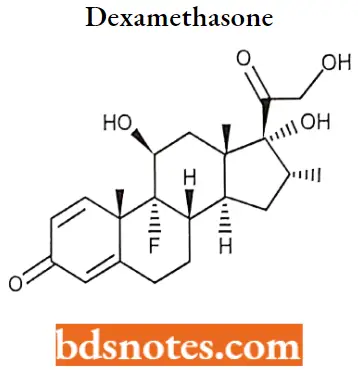
Dexamethasone MOA: Dexamethasone is a glucocorticoid agonist. Unbound dexamethasone crosses cell membranes and binds with high affinity to specific cytoplasmic glucocorticoid receptors.
- This complex binds to DNA elements (glucocorticoid response elements) which results in a modification of transcription and, hence, protein synthesis to achieve inhibition of leukocyte infiltration at the site of inflammation.
- Interference in the function of mediators of inflammatory response, suppression of humoral immune responses, and reduction in edema or scar tissue.
- The antiinflammatory actions of dexamethasone are thought to involve phospholipase A2 inhibitory proteins, and lipocortins, which control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes.
Dexamethasone Therapeutic Uses: It is used in the treatment of many conditions, including rheumatic problems, several skin diseases, severe allergies, asthma, chronic obstructive lung disease, croup, brain swelling, and along with antibiotics in tuberculosis.
In adrenocortical insufficiency, it should be used together with a medication that has greater mineralocorticoid effects such as fludrocortisone. In preterm labor, it may be used to improve outcomes in the baby.
Dexamethasone Adverse Reactions: The long-term use of dexamethasone may result in thrush, bone loss, cataracts, easy bruising, or muscle weakness. Acne, Insomnia, Vertigo, Increased appetite, weight gain, impaired skin healing, Depression, Euphoria, Hypertension, etc.
Thyroid And Antithyroid Drugs
The thyroid gland produces thyroid hormones. These Hormones are essential for life and have many effects on body metabolism, growth, and development. Thyroid hormones are derivatives of the amino acid tyrosine bound covalently to iodine. The two principal thyroid hormones are Thyroxine (also known as T4 or L-3,5,3′,5′-tetraiodothyronine).
- Triiodotyronine (T3 or L-3,5,3′-triiodothyronine)
- Thyroid hormones have two major physiological effects. They increase protein synthesis in virtually every body tissue and increase oxygen consumption dependent upon Na+-K+ATPsae (Na pump).
- Hyperthyroidism occurs seven to nine times more likely in women than in men. Hyperthyroidism often occurs between the ages of twenty and forty, while hypothyroidism often appears after the age of fifty.
- Certain types of thyroid tumors appear to be more prevalent in certain age groups. In the last few years, case studies of patients with hyperthyroidism in their youth have shown that, thirty years later, the same individuals have a greater tendency to develop hypothyroidism.
- Thyroid problems have a strong genetic component, and may “skip” generations. Family histories may be useful in suggesting the nature of thyroid problems at early stages.
- Prematurely gray hair, hair that starts to go gray before the age of thirty, is often a sign of thyroid dysfunction. Patchy hair loss, usually temporary, is often observed by persons with Graves’ or Hashimoto’s disease.
Hypothyroidism in Adults: It is a state of low serum thyroid hormone resulting from hypothalamic, pituitary, or thyroid insufficiency. Untreated, can lead to life-threatening myxedema coma (early signs of fatigue, forgetfulness, sensitivity to cold, unexplained weight gain.
Constipation progresses to decreasing mental stability, dry, flaky inelastic skin; puffy face, hands, and feet; hoarseness; periorbital edema; upper eyelid droop; dry, sparse hair Most common in women, incidence rising significantly in persons aged 40 to 50.
Hypothyroidism in Children (Cretinism): This is a Deficiency of thyroid hormone secretion during fetal development or early infancy that results in infantile cretinism (congenital hypothyroidism).
- Respiratory difficulties, persistent jaundice; and hoarse crying in infants; stunted growth (dwarfism), bone and muscle dystrophy, and mental deficiency in older children.
- 3 times more common in girls than in boys. Infants not treated within the first 3 months or children within two years suffer irreversible mental retardation.
Thyroiditis: Thyroiditis is the inflammation of the thyroid gland and can be divided into categories of autoimmune (Long-term inflammatory disease; Grave’s Disease, Hashimoto’s Disease).
Subacute granulomatous (Self-limiting inflammation), Riedel’s (rare, invasive fibrotic process), and miscellaneous (acute suppurative, chronic infective, chronic non-infective). More common in women than in men (5 to 7 times more common.)
Hyperthyroidism (Grave’s Disease, Basedow’s disease, Parry’s disease, thyrotoxicosis): Hyperthyroidism is a metabolic imbalance that results from thyroid hormone overproduction. The most common is Grave’s Disease. Only 5% of hyperthyroid patients are under age 15.
Grave’s Disease
Toxic goiter is a cause of 80% of hyperthyroidism. A diffusely enlarged thyroid gland associated with hyperthyroidism is known . as Grave’s disease. Autoimmune disease. The highest incidence is between the ages of 30 and 40.
Disorder of unknown etiology characterized by exophthalmos, enlarged pulsating thyroid gland, marked acceleration of heart rate, tendency to profuse sweats, nervous symptoms (fine muscular tremors, restlessness, irritability), psychic disturbances, emaciation, and increased metabolic rate.
Anti-thyroid Drugs
Anti-thyroid drugs (ATDs) are compounds that interfere with the body’s production of thyroid hormone, thereby reducing symptoms of hyperthyroidism.
ATDs were discovered accidentally in the mid-1940s when thiocyanate compounds used for heart disease were found to cause hypothyroidism. This led to the development of several compounds specifically tailored to reduce thyroid hormone production.
1. L-Thyroxine
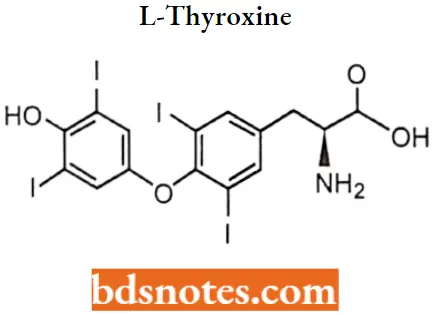
L-Thyroxine IUPAC name: (S)-2-Amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl] propanoic acid.
L-Thyroxine SAR:
- Levothyroxine is a synthetic form of thyroxine (T4), an endogenous hormone secreted by the thyroid gland, which is converted to its active metabolite, L-triiodothyronine (T3).
- T4 and T3 bind to thyroid receptor proteins in the cell nucleus and cause metabolic effects through the control of DNA transcription and protein synthesis.
L-Thyroxine SAR:
- The ether oxygen can be replaced isosterically by sulphur or methylene which also provide an angle of 120 °C. This change does not qualitatively impair the activity.
- A phenolic hydroxyl group at the 4′ position is important for hydrogen bonding to transport proteins. It can, however, be replaced by isosteric groups like NH2 or by a group that generates a hydroxyl group after metabolism (OCH3 or OCOCH3). However such a change results in reduced hormonal activity.
- The S’-position ortho to the hydroxyl and away from the other aromatic ring must be substituted by a lipophilic group. This can be a halogen (preferably Iodine) or any alkyl group approximately of the same size as Iodine such as an isopropyl group.
- The iodine atoms at 3 and 5 positions can be replaced by non-polar groups (such as methyl), as long as they keep the aromatic rings perpendicular to each other.
- The amino add side chain can be varied but should be para to the aromatic ring.
L-Thyroxine Metabolism: The primary pathway of thyroid hormone metabolism is through sequential deiodination. The liver is the main site of T4 deiodination, and along with the kidneys is responsible for about 80% of circulating T3.
In addition to deiodination, thyroid hormones are also excreted through the kidneys metabolized through conjugation and glucuronidation, and excreted directly into the bile and the gut where they undergo enterohepatic recirculation.
L-Thyroxine Therapeutic Use: Levothyroxine is typically used to treat hypothyroidism, and is the treatment of choice for people with hypothyroidism, who often require lifelong thyroid hormone therapy.
It may also be used to treat goiter via its ability to lower thyroid-stimulating hormone (TSH), a hormone that is considered goiter-inducing.
L-Thyroxine Adverse reactions: Adverse events are generally caused by incorrect dosing. Long-term suppression of TSH values below normal values will frequently cause cardiac side effects and contribute to decreases in bone mineral density (low TSH levels are also well known to contribute to osteoporosis).
- Too high a dose of levothyroxine causes hyperthyroidism. Overdose can result in heart palpitations abdominal pain, nausea, anxiousness, confusion, agitation, insomnia, weight loss, and increased appetite.
- Allergic reactions to the drug are characterized by symptoms such as difficulty breathing shortness of breath, or swelling of the face and tongue. Acute overdose may cause fever, hypoglycemia, heart failure, coma, and unrecognized adrenal insufficiency.
2. L-Thyronine
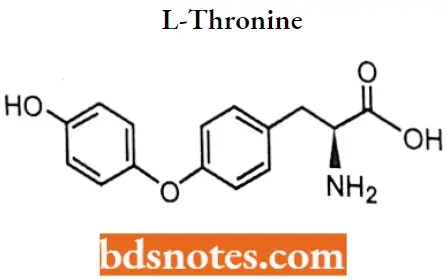
L-Thyronine IUPAC name: (2S)-2-amino-3-[4-(4-hydroxyphenoxy)phenyllpropanoic acid.
3. Propylthiouracil
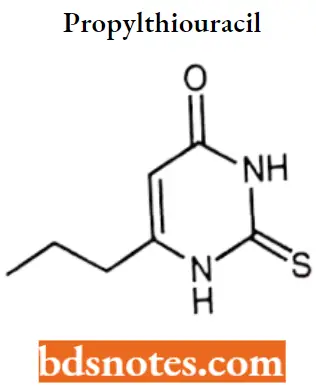
Propylthiouracil IUPAC name: 6-propyl-2-sulfanylpyrimidin-4-one.
Propylthiouracil MOA: Propylthiouracil binds to thyroid peroxidase and thereby inhibits the conversion of iodide to iodine. Thyroid peroxidase normally converts iodide to iodine (via hydrogen peroxide as a cofactor) and also catalyzes.
- The incorporation of the resulting iodide molecule onto both the 3 and or 5 positions of the phenol rings of tyrosines found in thyroglobulin.
- Thyroglobulin is degraded to produce thyroxine (T4) and tri-iodothyronine (T3), which are the main hormones produced by the thyroid gland. Therefore propylthiouracil effectively inhibits the production of new thyroid hormones.
Propylthiouracil Metabolism: Propylthiouracil is readily absorbed and is extensively metabolized. Approximately 35% of the drug is excreted in the urine, in intact and conjugated forms, within 24 hours.
Propylthiouracil Therapeutic Uses: Propylthiouracil (PTU) is a medication used to treat hyperthyroidism. This includes hyperthyroidism due to Graves’ disease and toxic multinodular goiter.
In a thyrotoxic crisis, it is generally more effective than methimazole. Otherwise, it is typically only used when methimazole surgery and radioactive iodine is not possible.
Propylthiouracil Adverse Reactions: Common side effects include itchiness, hair loss, swelling vomiting, muscle pains, numbness, and headache. Other severe side effects include liver problems and low blood cell counts.
Use during pregnancy may harm the baby. Propylthiouracil is in the antithyroid family of medications. It works by decreasing the amount of thyroid hormone produced by the thyroid gland and blocking the conversion of thyroxine(T4) to triiodothyronine(T3).
Methimazole
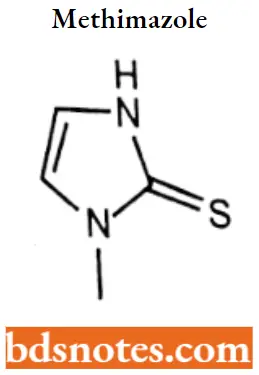
Methimazole IUPAC name: 1-Methyl-3H-imidazole-2-thione.
Methimazole MOA: It inhibits the enzyme thyroperoxidase, which normally acts in thyroid hormone synthesis by oxidizing the anion iodide (I-) to iodine (12), hypoiodous acid (HOI).
Enzyme-linked hypoioctate (EOI) facilitates iodine’s addition to tyrosine residues on the hormone precursor thyroglobulin, a necessary step in the synthesis of triiodothyronine (T3) and thyroxine (T4)
Methimazole Metabolism: Primarily hepatic. Metabolized rapidly, requiring frequent administration.
Methimazole Therapeutic Uses: Methimazole is a drug used to treat hyperthyroidism such as in Graves’ disease, a condition that occurs when the thyroid gland begins to produce an excess of thyroid hormone.
The drug may also be taken before thyroid surgery to lower thyroid hormone levels and minimize the effects of thyroid manipulation.
Methimazole Adverse reaction: It is important to monitor any symptoms of fever or sore throat while taking thiamazole; this could indicate the development of agranulocytosis, an uncommon but severe side effect resulting from a drop in the white blood cell count (to be specific, neutropenia, a deficiency of neutrophils).
A complete blood count (CBC) with differential is performed to confirm the suspicion, in which case the drug is discontinued.
Drugs Acting On Endocrine System Multiple Choice Questions And Answers
Question 1. Hormone production is a function related to ___________.
- Hypothalamus
- Pons
- Hippocampus
- Medulla
Answer: 1. Hypothalamus
Question 2. The mode of action of steroid hormones involves _____________.
- A second messenger
- Modification of
- Stimulation of DNA replication
- Stimulation of mRNA enzyme activity
Answer: 4. Stimulation of mRNA enzyme activity
Question 3. Prostaglandins are local regulators whose basic structure is derived from __________.
- Oligosaccharides
- Fatty acids
- Steroids
- Amino acids
Answer: 2. Fatty acids
Question 4. One reason a person might be several overweight is due to __________.
- An under-secretion of thyroxine
- A defect in hormone release from the posterior pituitary
- A lower-than-normal level of insulin-like growth factors
- Hyposecretion of oxytocin
Answer: 1. An under-secretion of thyroxine
Question 5. All of the following are steroid hormones except ________.
- Androgen
- Cortisol
- Estrogen
- Insulin
Answer: 4. Insulin
Question 6. Testosterone is an example of __________.
- An androgen
- An estrogen
- A progestin
- A catecholamine
Answer: 1. An androgen
Question 7. In children, hypothyroidism (underactive thyroid gland) can lead to _________.
- Goiter
- Acromegaly
- Cretinism
- Myxedema
Answer: 3. Cretinism
Question 8. In Males, the sex hormone that maintains sexual organs and secondary sex characteristics is ________.
- Progesterone
- Estrogen
- Testosterone
- Relaxin
Answer: 3. Testosterone
Question 9. Anabolic steroids are ________ versions of testosterone.
- Effective
- Synthetic
- Natural
- Ineffective
Answer: 2. Synthetic
Question 10. Which of the following is characteristic of Male Erectile Disorder?
- A failure to attain an erection from the outset of sexual activity
- First experiencing an erection but then losing tumescence before penetration
- Losing tumescence during penetration but before orgasm
- All of the above
Answer: 1. A failure to attain an erection from the outset of sexual activity
Drugs Acting On Endocrine System Short Questions And Answers
Question 1. What is the endocrine system?
Answer:
Many different glands make up the endocrine system. The hypothalamus, pituitary gland, and pineal gland are in your brain. The thyroid and parathyroid glands are in your neck.
The thymus is between your lungs, the adrenals are on top of your kidneys, and the pancreas is behind your stomach. Your ovaries (if you’re a woman) or testes (if you’re a man) are in your pelvic region.
Question 2. What is estrogen? and give therapeutic uses of it.
Answer:
Estrogen, or estrogen, is the primary female sex hormone. It is responsible for the development and regulation of the female reproductive system and secondary sex characteristics.
- Three major endogenous estrogens in females have estrogenic hormonal activity: estrone, estradiol, and estriol. Estrogen is a type of medication that is used most commonly in hormonal birth control and menopausal hormone therapy.
- They can also be used in the treatment of hormone-sensitive cancers like breast cancer and prostate cancer and for various other indications. Estrogens are used alone or in combination with progestogens.
Question 3. What are Atv anabolic steroids?
Answer:
Anabolic steroid (a muscle-building chemical) occurs naturally in the human body, but only in tiny quantities.
- It is very similar in structure to the male hormone testosterone, and has many of the same effects in terms of increasing muscle mass, without some of the more unwanted side-effects For Example. Nandrolone esters an used clinically.
- Although increasingly rarely, for people in catabolic states with major bums, cancer, and AIDS, an ophthalmological formulation was available to support cornea healing.
Question 4. Give SAR points for testosterone.
Answer:
- It lacks the 2-carbon side chain attached to the 17 position, making it a 19-carbon steroid (an androstane).
- 17 alpha methyl group increases the duration of action and improves bioavailability. But this change increases hepatotoxicity.
- The esterification of the 17 beta OH group increases the duration of action and bioavailability.
- The removal of 19th carbon led to more anabolic selective molecules and less androgenic selectivity.
5. What are oral contraceptives?
Answer:
Oral contraceptives, also called birth control pills, are a safe and reliable option for preventing unwanted pregnancy. Most oral contraceptives contain a combination of 2 types of hormones: estrogen and progestin.
Both of these hormones are naturally found in women’s bodies. There are many different types of estrogens and progestins, and different types of pills contain different combinations, but they all work similarly. Some pills contain only progestin, sometimes called the “mini-pill.”

Leave a Reply