Direct Filling Gold
Prior to the discovery of amalgam, pure gold was very popular as a filling material. The use of gold foil in modern dentistry starts around 1400 in renaissance Italy. Its use is described in a medical text written in 1425 by Giovanni Arcolani (Johannas D’Arcola) a professor of the University of Bologna. Robert Woofendale is credited to have introduced it to the USA in 1766 on his arrival from England. However, its use became widespread in the USA only towards the beginning of the 19th century.
Read And Learn More: Basic Dental Materials Notes

It is the most noble of metals and rarely tarnishes in the oral cavity. Gold in its pure form is very soft (25 BHN). Its malleability and lack of a surface oxide layer permit increments to be welded together. This unique characteristic of gold to be welded at room temperature (cold-welded), allows gold to be used as a direct filling material.
Although the dental profession sometimes refers to all direct filling golds (DFGs) as ‘Gold Foils’ the present products may be divided into three categories. All are of 99.99% or higher purity, except two (Platinized foil and Electraloy RV).
Currently, direct filling gold is not as widely used as it once was. However, it will continue to be described because it is still used and is an excellent restorative material when placed properly.
Direct Filling Gold Applications
- Pits and small class 1 restorations.
- For repair of casting margins.
- For class 2, class 5 and class 6 restorations.
- Repair of cement vent holes and perforations in gold crowns.

Direct Filling Gold Contraindications
- Teeth with very large pulp chambers.
- Periodontally compromised teeth.
- Handicapped patients who are unable to sit for the long dental appointments required for the procedures.
- Root canal filled teeth because these teeth are brittle.
Direct Filling Gold Types
Many categories of direct filling gold are available and is based on its physical form and manufacturing process.
- Foil (fibrous gold)
- Sheet
- Cohesive
- Noncohesive
- Ropes
- Cylinders
- Laminates
- Platinized
- Sheet

- Electrolytic precipitate (crystalline gold)
- Mat
- Mat foil
- Gold-calcium alloy
- Granulated gold (encapsulated powder)
Direct Filling Gold Composition and purity
- Most modern gold restorations are made of gold of high purity (99.99 % or higher) excepting for the platinized and alloyed gold.
Gold Foil
Gold foil is the oldest of all products described. It was manufactured for dental applications as early as 1812 by Marcus Bull (USA). It is manufactured by beating gold into sheets.
Gold Foil Manufacture
- Gold is malleable. A cast ingot of 15 mm thickness is beaten to a submicroscopic thickness of 15 or 25 µm. The product is called gold foil. The crystals of the original cast metal are deformed and elongated so that they have a fibrous structure, hence it is also known as fibrous gold.
Gold Foil Supplied As
- Flat square sheets (of varying thickness) in booklet form, 12 sheets (each 10 cm × 10 cm) per booklet.
- No. 4 wt. 4 grains (0.259 gram) 0.51 µm thick.
- No. 3 wt. 3 grains (0.194 gram) 0.38 µm thick.
The number denotes the weight of the gold. Other sheets also available are Nos. 20, 40, 60 and 90. No. 3 foil is used to manufacture electrolytic and powder gold.
- Preformed cylinders and ropes. These are made by cutting the No. 4 gold foil sheets into strips of varying widths (3.2, 4.8, etc.) and then rolling and compressing them into pellets or cylinders. They may be ‘carbonized’.
- A number of sheets of foil may be placed one top of each other to form laminated gold foil. One type of laminated foil is the platinized foil, which is a sheet of pure platinum foil sandwiched between two sheets of pure gold foil.
Gold Foil Carbonized or corrugated gold
- Rolling into a pellet or cylinder form is convenient for carrying and compacting into cavities. Many dentists cut and roll their own gold. Preformed gold foil in the form of ropes and cylinders may be available in ‘carbonized’ or ‘corrugated’ form (made from No. 4 foil that has been ‘carbonized’ or ‘corrugated’). This form of gold foil is of historical interest because it was an outcome of the great Chicago fire in 1871. Corrugated foil is obtained by placing the gold foil in between sheets of paper and igniting it in a closed container. On igniting, the paper gets charred, but the gold foil is left unharmed except that it becomes ‘corrugated’. This is because of the shriveling of the paper while oxidizing in the airtight safe. After the carbon is removed it is found that the gold exhibits superior welding property.
Gold Foil Platinized foil
- This is a laminated foil in which pure platinum foil is sandwiched between two sheets of No. 4 gold foil. The sheets are beaten and joined together. Platinum is added to gold foil to increase the hardness of the restoration. This product is available only in No. 4 sheet form.
Cohesive and noncohesive gold
- In practice only the sheet foil is furnished in both conditions, though all forms of direct filling gold could be supplied in cohesive and noncohesive form.
Noncohesive The earliest gold foils were ‘noncohesive’ in nature. The manufacturer subjects the foil to a volatile agent such as ammonia, which is absorbed on the surface of the gold. Ammonia-treated foil is called noncohesive foil. This adsorption of ammonia gas onto the surface of the gold prevents the gold foil from adhering if it folds back on itself, or contacts other pieces of foil. This is also useful when pellets or cylinders are packed together in containers. It prevents premature cohesion of pellets in their container. It also protects the surface of the gold from contamination which can’t be removed. This treatment enables the user of the foil to handle it with a little more ease.
- Noncohesive gold can also have adsorbed agents like iron salt or an acidic gas (sulfur or phosphorous containing groups) on its surface. The volatile film is readily removed by heating (see annealing), thereby restoring the cohesive character of the foil. Noncohesive gold is rarely used currently, but may be used to build-up the bulk of a direct gold restoration.
- Disadvantage of noncohesive gold Because the gold wouldn’t stick to itself one could not build contour above the cavosurface. Fillings were pretty much flat from cavosurface to cavosurface.
Cohesive Cohesive gold is also called ‘sticky gold’. It was introduced in 1854 by Dr. Robert Arthur when he published his paper on sticky gold (cohesive gold), “A New Method of Using Gold Foil”.
- For cold-welding, gold should have a clean surface free from impurities. Gold attracts gases, e.g. oxygen to its surface and any absorbed gas film prevents cohesion of individual increments of gold during their compaction. The manufacturer, therefore, supplies the gold essentially free of surface contaminants. This type of gold is known as cohesive gold foil.
Representative commercial products Jensen Gold Foil
Electrolytic Precipitate
Crystalline gold powder is formed by electrolytic precipitation. The powder has a dendritic crystalline structure. The powder is formed into shapes or strips by sintering (heat fusion) at high temperatures but below their melting point. The particles coalesce or join together at this temperature.
Electrolytic Precipitate Available As
- Mat, mat foil and alloyed.
Electrolytic Precipitate Mat Gold
- Mat gold is electrolytically precipitated gold sandwiched between sheets of foil and then formed into strips. The strips can be cut by the dentist into the desired size. Mat gold is used to build-up the bulk of the restoration, as it can be more easily compacted and adapted to the cavity. However, mat gold has lots of voids and results in a pitted external surface. Therefore, foil gold is used to cover the mat gold and form the surface of the restoration.
Electrolytic Precipitate Mat Foil
- It is a sandwich of electrolytic precipitated gold powder between sheets of No. 3 gold foil. The sandwich is sintered and cut into strips of differing widths. The dentist can then cut these to the desired lengths. Sandwiching mat between foil sheets was done to try to eliminate the need to veneer the restoration with a layer of foil. This type is no longer marketed.
Electrolytic Precipitate Alloyed Electrolytic Precipitates
- A form of electrolytic gold is an alloy of gold and calcium (0.1% by wt.) called ‘Electraloy RV’. For greater ease of handling, the alloy is sandwiched between two layers of gold foil. Calcium produces stronger restorations by dispersion strengthening, which locks in cold work strengthening. Thus, alloying with calcium changes the crystalline structure and makes it harder and stronger.
Powdered Gold
Synonym Granular gold
Since the 19th century, chemically precipitated gold powders have been available in agglomerated form with a liquid such as alcohol or dilute carbolic acid, which held the agglomerate together. The agglomerates usually disintegrated when compaction was attempted, so the gold powder was mixed with a wax binder and enclosed in a No. 3 gold foil. This form was first marketed commercially in the 1960s as ‘Goldent’ (Morgan Hastings Co.). In the 1980s another brand ‘EZ Gold’ (Ivoclar North America) was introduced.
Powdered Gold Manufacture
- A fine powder is formed by chemical precipitation or by atomizing the metal. The particle sizes vary (average 15 µm). The pellets are mixed with soft wax (0.01% organic wax, which is burnt off prior to use) and then wrapped with gold foil (No. 3), rather than sintering the mass, like for mat gold.
Powdered Gold Available As
- The powdered gold pellets have a cylindrical or irregular shape and a diameter of 1 to 2 mm. The ratio of gold foil to powder varies from 1 to 3 for the smallest pellets to approximately 1 to 9 for the largest.
- The foil acts as an effective container and matrix for the powdered metal, while it is condensed. Some operators believe that the use of powdered gold pellets increases cohesion during compaction and reduces the time required for placing the restoration. This is because each pellet contains 10 times more metal by volume than comparable sized pellet of gold foil.
Powdered Gold Manipulation Of Direct Filling Gold
There are three processes involved
- Desorbing
- Compaction
- Finishing
Desorbing Or Degassing
Synonym Degassing, annealing
- The direct filling golds are received by the dentist in a cohesive condition, except for the noncohesive golds. However, during storage and packaging, they absorb gases from the atmosphere. Adsorbed gases prevent gold from fusing. Hence, it is necessary for the dentist to heat the foil or pellet immediately before it is carried into the prepared cavity. This heating process which removes surface gases (oxygen, nitrogen, ammonia, moisture or sulfur dioxide) and ensures a clean surface is called desorbing or degassing (rather than annealing). Storage in air tight containers is advised and the operator should wear chamois finger tips to protect the gold from contamination. A totally dry cavity is essential throughout the compaction process in order to allow complete cohesion.
- Direct filling golds may be heated by one of two methods
- In bulk on a tray by gas-flame or electricity.
- Piece by piece, in a well-adjusted alcohol flame.
- In practice, all but powder gold may be desorbed on a tray. Powder gold must be heated in a flame to ensure the complete burning away of the wax.
- Precaution during bulk heating Excessive amounts should be avoided, since the difficulties arising from prolonged heating can arise from repeated heating as well. Care should be taken to handle pieces with stainless steel wire points or similar instruments that will not contaminate the gold.
Electric annealing
The electric ‘annealer’ is maintained at a temperature between 340 °C and 370 °C. The time required varies from 5 to 20 minutes depending on the temperature and the quantity of gold on the tray.
Problems associated with electric annealing are
- Pellets may stick together, if the tray is moved.
- Air currents may affect the uniformity of heating.
- Difficult to anneal appropriate amounts of gold.
- Over sintering.
- Greater exposure to contamination.
- Size selection among the pieces of desorbed gold is limited.
To prevent their sliding and sticking together, the tray of a recent electric annealer (Neibert electric gold foil annealer) provides individual compartments for each piece of foil.


Flame desorption
Each piece is picked individually by a clean sharp pointed nonoxidizing instrument (nickel chrome, stainless steel, iridioplatinum, etc.), heated directly over the open flame, and placed in the prepared cavity. The fuel for the flame may be alcohol or gas. Alcohol is preferred as there is less danger of contamination. The alcohol should be pure methanol or ethanol without colorants or other additives.
Advantages of flame desorption are
- Ability to select a piece of gold of the desired size.
- Desorption of only those pieces used.
- Less exposure to contamination between degassing and use.
- Less danger of oversintering.
Under-heating does not remove impurities well and results in incomplete cohesion. Carbon deposited by the flame can cause pitting and flaking of the surface.
Overheating leads to oversintering and possibly contamination from the tray, instruments or flame. This results in incomplete cohesion, embrittlement of the portion being heated and poor compaction characteristics. Overheating can result from too long a time even at a proper temperature or from too high a temperature.
Compaction
The gold may be compacted by
- Hand mallet
- Pneumatic vibratory condensers
- Electrically driven condensers
Hand mallet
Earlier gold was compacted entirely with a mallet. Starting points are cut in the prepared cavity. The first pieces of foil are wedged into these areas and compacted. The condenser is placed against the foil and struck sharply, with a small mallet. Subsequently additional foil is wedged in the same manner, till the cavity is filled. Each increment of gold must be carefully ‘stepped’ by placing the condenser point in successive adjacent positions. This permits each piece to be compacted over its entire surface so that voids are not bridged.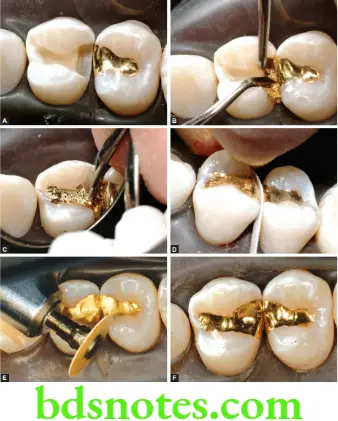

Condensers
- The original foil condensers had a single pyramid-shaped face, but current instruments have a series of small pyramidal serrations on the face. These serrations act as swaggers, exerting lateral forces on their inclines in addition to providing direct compressive forces. They also cut through the outer layers to allow air trapped below the surface to escape.
- Size of the condenser point This is an important factor in determining the effectiveness of compaction. Small condenser points compact without using forces that might damage oral structures. The diameter of circular points should be 0.5 mm and 1 mm.
Mechanical condensers
- Electromagnetic or spring-loaded (not used now a days), pneumatic (compressed air powered) and electric mallet have provided a mechanical means of applying force. The mechanical devices consist of points activated by comparatively light blows that are repeated with frequencies that range from 360–3600/minute.
- Advantages Faster and more comfortable for the patient.
Finishing
- As with amalgam the cavity is slightly overfilled. The surface is probed with a explorer to test for proper compaction and to make sure all voids are eliminated. If the probe penetrates easily, condensation is continued with more force. The excess is removed with a gold file or burs. The surface is burnished with a ball burnisher to strain harden the surface. Final polish (optional) is achieved with sof-lex disks or other commercially available gold polishing kits.
Properties Of Compacted Gold
Strength
- The greatest strength is in the most dense area and the weakest part is the porous area, where layers or crystals are not closely compacted. In direct filling gold, the failure occurs from tensile stress, due to incomplete cohesion. Thus transverse strength is a measure of cohesion.
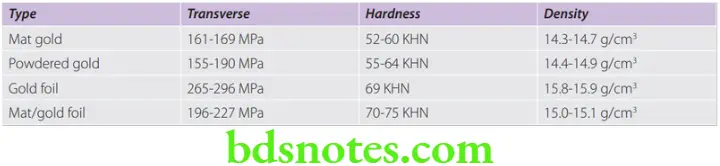
Hardness
- The hardness indicates the overall quality of compacted gold. A reduction in hardness probably indicates the presence of porosity.
Density
- True density of pure gold is 19.3 g/cm3. However in DFGs this is not achieved, because it is not possible to eliminate voids completely during compaction. Thus density of DFGs is usually less than ideal.
- The transverse strength, hardness and (apparent) density are somewhat greater when gold foil is used alone or in combination with mat gold, as compared with other forms. The difference in physical properties among the various forms of gold including the gold-calcium alloy and the method of compaction are not clinically significant. The physical properties are probably more greatly influenced by the competence of the operator in manipulating and placing the gold.
Effect of Voids
- The amount of voids is estimated by the apparent density of compacted gold. Voids on the restoration surface (pits) increase the susceptibility to corrosion and deposition of plaque. Voids at the restoration-tooth interface may cause gross leakage and secondary caries development (in properly compacted gold, microleakage is minimum).
Tarnish and Corrosion
- Resistance to tarnish and corrosion is good, if compacted well.
Biocompatibility
- The pulpal response is minimal if compacted well. The technique, however, does involve a certain amount of trauma to the tooth and its supporting tissues. In smaller teeth, this is an important consideration. The mechanical condenser causes less trauma than the manual technique.
Advantages And Disadvantages
Advantages
- Esthetic because of its gold color and high lustre.
- Tarnish and corrosion resistant.
- Good mechanical properties.
- Good biocompatibility.
Disadvantages
- Nonesthetic because it is not tooth colored.
- High CTE (coefficient of thermal conductivity).
- Problems of temperature sensitivity if not insulated with a base
- Manipulation is technically challenging.
The technical skill of the dentist is very important for the success of the direct gold restoration. A gold restoration of poor quality can be one of the most inferior of all restorations.

Leave a Reply