Dental Cement
Base: Layer of insulating, sometimes medicated, cement, placed in the deep portion of the preparation to protect pulpal tissues from thermal and chemical injury.
Cavity liner: A thin layer of cement, such as calcium hydroxide suspension in an aqueous on resin carrier, used for protection of the pulp, certain glass ionomer cement that is used as an intermediate layer between tooth structures and composite restorative materials are also considered liners.
Atraumatic restorative treatment: Clinical procedures performed without dental laws, air/water spray, or anesthesia that consists of manual excavation of various tissues and restoration of the tooth cavity with a type of fluoride-releasing cement.
Read And Learn More: Basic Dental Materials Notes
Cermet: Glass ionomer cement that has been reinforced with filler particles prepared by fusing silver particles into glass.
Craze: Network of fine, interconnected cracks formed within the surface of aqueous-based cement as a result of rapid dehydration.
Luting agent: A viscous material placed between a tooth structure and a prosthesis that hardens through chemical reactions to firmly attach the prosthesis to the tooth structure.
Restoration: Filling material or prosthesis used to restore or replace a tooth, a portion of a tooth, or multiple teeth on other oral tissues.
Varnish: a solution of natural gum, synthetic resins, on resins dissolved in a volatile solvent, such as acetones others on chloroform.
Zinc Phosphate Cement (ADA 8) [Spotter]
![Dental cements Zinc Phosphate Cement (ADA 8) [Spotter]](https://bdsnotes.com/wp-content/uploads/2023/07/Dental-cements-Zinc-Phosphate-Cement-ADA-8-Spotter-2.png)
Composition:

![Dental cements Zinc Phosphate Cement (ADA 8) [Spotter]](https://bdsnotes.com/wp-content/uploads/2023/07/Dental-cements-Zinc-Phosphate-Cement-ADA-8-Spotter-1.png)
Application:
- Luting of Restorations
- Temporary restoration
- High strength bases
Trade names:
- Harvard, Confit
Zinc Oxide Eugenol Cement (ADA 30) [Spotter]
![Dental cements Zinc Oxide Eugenol Cement (ADA 30) [Spotterr]](https://bdsnotes.com/wp-content/uploads/2023/07/Dental-cements-Zinc-Oxide-Eugenol-Cement-ADA-30-Spotterr.png)
![Dental cements Zinc Oxide Eugenol Cement (ADA 30) [Spotter]](https://bdsnotes.com/wp-content/uploads/2023/07/Dental-cements-Zinc-Oxide-Eugenol-Cement-ADA-30-Spotter.png)
Composition:
![Dental cements Zinc oxide eugenoul Cements [Spotter]](https://bdsnotes.com/wp-content/uploads/2023/07/Dental-cements-Zinc-oxide-eugenoul-Cements-Spotter.png)
Glass Ionomer Cements [Spotter]
![Dental cements Glass Ionomer Cements [Spotter].](https://bdsnotes.com/wp-content/uploads/2023/07/Dental-cements-Glass-Ionomer-Cements-Spotter.png)
Trades names
- Aqua cement
- Fuji
- Chem Fil
Composition
![Dental cements Glass Ionomer Cements [Spotter].](https://bdsnotes.com/wp-content/uploads/2023/07/Dental-cements-Glass-Ionomer-Cements-Spotter-2.png)
Zinc Polycarboxylate Cement [Spotter]
Composition:

Applications:
- Luting permanent restorations
- Bases and liners
- Root canal fillings
Calcium Hydroxide [Spotter]
Relatively weak cement employed for direct & indirect pulp capping agent
Other uses:
High strength base.
Composition:
Base: Glycol salicylate, Ca So4, Ti O2, Ca Tungstate
Catalytic paste: Ca (OH) 2, Zinc Oxide, Zinc Stearate, Sulfonamide
⇒ \(\text { Commercial Types }\left\{\begin{array}{l}
\text {Self-cured – Dycol life care } \\
\text { Light cured – Prisma }
\end{array}\right.\)
Cavity Varnish [Spotter] Definition:
Is a solution of one or more resins which when applied onto the cavity wells, evaporates leaving a thin resin film that serves as a barrier between the restoration and the dentinal tubules.
Cavity Varnish Applications:
- Reduces microleakage around the margins of newly formed amalgam.
- Reduces passage of irritants into the dental tubules
- Surface coating over certain restoration.
![Dental cements Cavity Varnish [Spotter]](https://bdsnotes.com/wp-content/uploads/2023/07/Dental-cements-Cavity-Varnish-Spotter.png)
Cavity Varnish Composition:
- Natural gum such as copal, Rosin, or Synthetic resin,
- Medical agents – Chlorbutanol, Thymol, Eugenol.
Dental Cement
Dental cements are materials of multiple uses including restorations, luting, and therapeutic. They are generally materials of comparatively low strength but have extensive use in dentistry. The first dental cement is said to have been introduced in 1785 by Sorel who created the ‘zinc-oxide-chloric-cement’.
Nearly a hundred years later Rostain and then Fleck developed and introduced the zinc phosphate cement. Around the same period, silicates were also developed.
Cements have come a long way since then. Many of them have been improved considerably, while some like the silicate cement have been discontinued. Some like the glass ionomers and the polycarboxylate have adhesive properties and form a chemical bond to dentin and enamel. Regardless of some inferior properties, they possess so many desirable features that they are widely used in dentistry.
Dental Cement Classification Cement has a wide variety of uses, properties, and reaction mechanisms. This makes them generally difficult to classify.
ISO Classification
- Water-based cement Zinc phosphate, glass ionomer, etc.
- Oil-based cement ZOE and noneugenol cement
- Resin or polymer-based cements Resin cement, compomer, etc.
According to the setting reaction The materials may be classified as
- Acid-base reaction cements
- Polymerizing cements
- Dual cure cements
- Tricure cements
Acid-base reaction cements are formulated as powder and liquid. The liquid acts as the acid and the powder as the base. On mixing the two an acid-base reaction takes place resulting in a viscous paste, which hardens to a solid mass.
Polymerizing cements These cements are set by polymerizing reactions which may be light-activated or chemically activated, e.g. resin cements.
Dual and Tricare Cement Dual cure cement is set by acid-base and any one of the polymerization (light-activated or chemically activated) mechanisms. Tricure cement utilizes all three mechanisms for hardening.
Classification of cement based on application (ISO 9917-1:2007)
- Luting
- Bases or lining
- Restoration
General Structure
On mixing the powder and liquid, only a part of the powder reacts with the liquid, and the final set material is composed of
- A core of unreacted powder, surrounded by
- A matrix is formed by the reaction product of the powder and the liquid.
Uses Of Cement Cement have a wide variety of uses in dentistry.
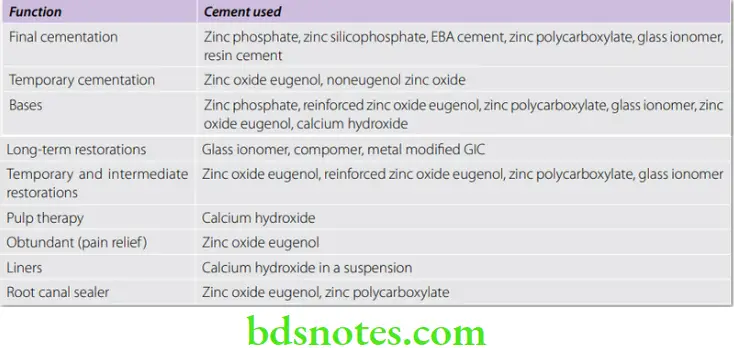
General Properties Of Cement
Though cements are formulated to serve a variety of functions, the two most common applications of dental cements are luting and restorations. Some of the minimum requirements for water-based dental cement.
Net Setting Time
Net setting time is the period of time, measured from the end of mixing until the material has set (as per ISO criteria).
Strength
- Most cements are comparatively weak when compared to restorative materials like amalgam and composites. The strength required depends on the application. For example, cement used as a base under amalgam should have sufficient strength to withstand condensation forces.
- Many dental cements as well as restorative materials continue to gain strength with time. For this reason, patients are often advised to wait at least 2 hours before any food is placed in the mouth. In addition, the side in which the restoration has been placed is avoided for a further 24-hour period.
Modulus Of Elasticity (MOE)
This is a measure of the stiffness of the cement. Cement under ceramic crowns should have sufficient stiffness to withstand masticatory loads. A low MOE can result in flexing of the restoration resulting in fracture.
Solubility And Disintegration
- This is an important property as it can determine the long-term survivability of restorations. Solubility and disintegration of the cement at the margins can eventually lead to problems like inflammation, caries, sensitivity, etc.
- Most cements exhibit varying degrees of solubility. ISO specification No. 99171:2007 uses in vitro testing with 0.1 mol/L lactic acid with a pH of 2.74. However, a more reliable test would be an in vivo test as conditions in the mouth are far more complex.
- Solubility and disintegration can be reduced by proper manipulation, minimizing the exposure of the cement to the oral environment, and protecting the cement during setting and the initial 24-hour period.
Film Thickness
Film thickness is an important property, especially for luting cements. A thinner film is more advantageous for luting.

- It improves the seating of the restoration.
- It helps in greater flow and wetting of the tooth and restoration surface, thus improving bonding.
- It minimizes the air spaces and structural defects present in the bulk of the cement.
Film thickness is measured in µm. ISO 9917-1:2007 specifies the maximum film thickness for luting cement as 25 μm.
Biological Properties
- Most cements are placed within the dentin and in many instances in close proximity to the pulp. Thus it is important that the cement should not be irritant or toxic to the pulp.
- PH of the cement Most cement is acidic. The exceptions are zinc oxide eugenol, calcium hydroxide, and resin cement. The acidity of cement is higher at the time of placement but gradually decreases with time.
- Pulpal response The pulp response may be classified as mild, moderate, or severe. Originally silicate cement was used as a reference to compare the pulpal response to various cements. Because of its high acidity, silicates were classed as severe irritants. High acidity can irritate and sometimes lead to irreversible pulpal damage. In some patients, it can cause severe pain and sensitivity. Monomer present in resin-based cement is also a potential irritant.
- Pulp protection In case of deep cavities and where the cement is classed as an irritant measures to protect the pulp are indicated.
- Avoid thin mixes.
- Pulp protection should be carried out in deep cavities through the use of an intervening liner or base.
Fluoride Release
Many cements contain fluoride which is gradually released over a period of time to impart adjacent teeth structure with caries resistance. Glass ionomer is an example of a fluoride-releasing cement.
Fluoride recharging
The process by which a restorative material, specifically glass ionomer cement, absorbs fluoride from a solution with a high fluoride concentration.
Silicate Cements
Silicate cements are said to have been introduced in 1873 by Fletcher as an anterior esthetic filling material. They were translucent and resembled porcelain in appearance. Though the initial esthetics were satisfactory, over a period of time silicates degraded and stained. Leakage around the margins results in dark margins. Silicates are attacked by oral fluids and in time degrade.

- The average life of a silicate restoration is four years. Some may last as long as 25 years, others may require replacement in a year or even less.
- The incidence of secondary caries is markedly less around silicate restorations. This is surprising when considering that severe leakage takes place at its margins. Also, the incidence of contact caries is less when compared to amalgam restorations (contact caries is the term applied to caries occurring on the proximal surface of the tooth adjacent to the restoration).
- The anticariogenic property is due to the presence of 15% fluoride.
- Fluoride release is slow and occurs throughout the life of the restoration. Silicate cements were classed as a severe irritant to the pulp because of its low pH (acidic). For many years silicate served as a standard for comparing the pulpal response to other materials. In deep cavities, the pulp had to be protected with varnish or calcium hydroxide.
- With the development of better alternate materials like composite resin and glass ionomer cement, silicates gradually fell out of favor. By the 1980s and 1990s, they were gradually phased out of the market and are rarely used. However, silicate cements are of historical interest as they were the first tooth-colored filling materials. It also forms the basis for the glass ionomer system.
Zinc Phosphate Cement
Zinc phosphate is the oldest of the luting cement and thus serves as a standard with which newer cement can be compared. The terms ‘Crown and Bridge’ and ‘Zinc Oxyphosphate’ have also been used for this cement.
Zinc Phosphate Cement Applications
- Luting of restorations (inlays, crowns, fixed dental prostheses, etc.)
- High strength bases.
- Temporary restorations.
- Luting of orthodontic bands and brackets.
Zinc Phosphate Cement Classification ISO 9917-1:2007 designates them as
- Luting (Maximum film thickness—25 μm)
- Bases and lining
Zinc Phosphate Cement Available As
- Powder and liquid system.
- Capsules of preproportioned powder and liquid.
Supplied in shades of yellow, gray, golden brown, pink and white.
Representative commercial products Confit, Harvard, Zinc Cement (DPI), Modern Tenacin, Poscal (VOCO), De Trey Zinc (Dentsply), Hy Bond, etc.
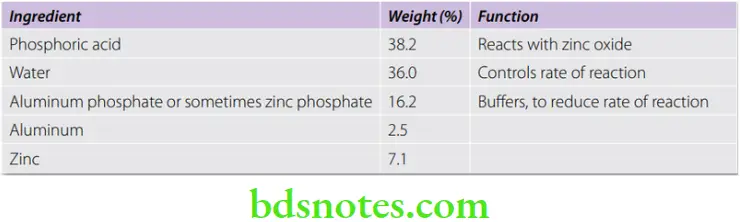
Zinc Phosphate Cement Composition
Powder


Liquid
Zinc Phosphate Cement Manufacture
- The ingredients are mixed and heated at temperatures between 1,000 °C and 1,400 °C (sintering). After sintering, the cake formed is cooled quickly. This causes the material to crack which helps in grinding of the material to a fine powder. This process is known as fritting.
- The liquid is produced by adding aluminum and sometimes zinc or their compounds into an orthophosphoric acid solution.
Setting Reaction
- When the powder is mixed with liquid, phosphoric acid attacks the surface of the particles and releases zinc ions.
- The aluminum in the liquid is essential for cement formation. The aluminum complexes with the phosphoric acid and the zinc ions to form a zinc aluminophosphate gel. The reaction is exothermic.
Structure Of Set Cement
The set cement has a cored structure consisting primarily of unreacted zinc oxide particles embedded in a matrix of zinc aluminophosphate.
Net Setting Time
According to ISO 9917-1:2007, the net setting time can vary from 2.5 to 8 minutes for luting and 2.5 to 6 for base and lining type.
Control of setting time
Manufacturing process
- Sintering temperature The higher the temperature, the more slowly the cement sets.
- Particle size Finer particles react more quickly as a greater surface area is exposed to the liquid.
- The water content of liquid Presence of excess water accelerates, whereas insufficient water retards the reaction.
- Buffering agents When added slow down the reaction.
Factors under control of the operator
- Temperature Higher temperatures accelerate the reaction. Cooling the mixing slab is an effective way of slowing the reaction and prolonging the working time.
- Powder-liquid ratio More the liquid, the slower the reaction.
- Rate of addition of powder to liquid The reaction is slower if the powder is incorporated slowly.
- Mixing time The longer the mixing time (within practical limits), the slower is the rate of reaction.
Properties
Compressive Strength
- The fully set zinc phosphate cement has a relatively high compressive strength ranging from 104 to 119 MPa. The set cement gains approximately 70% of its maximum strength in the first 30 minutes. The strength continues to rise with time and maximum strength is attained at the end of 24 hours.
- The strength of zinc phosphate cement is sufficient when used as a base or luting agent. However, when it is exposed to the oral environment, e.g. temporary restorations, its brittleness and low strength cause it to fracture and disintegrate. Also, prolonged contact with oral fluids or water gradually reduces its strength. This may be due to the slow dissolution of the cement.
- Factors affecting strength
- Powder-liquid ratio More the powder, the greater the strength.
- The water content of the liquid Both loss and gain, reduces the strength.
Tensile Strength
The set cement is weaker in tension (5.5 MPa), thus making it brittle.
Modulus Of Elasticity (Stiffness)
It is comparatively high (13.7 GPa). This makes it stiff and resistant to elastic deformation. This is beneficial when it is used to cement restorations that are subjected to high masticatory stresses.
Solubility And Disintegration
- This property is important for cements used for permanent cementation. When tested according to ISO specification, the maximum solubility permitted is 0.3.
- However, in the mouth, they show greater disintegration over a period of time. This shows that other factors are involved (like wear, abrasion, chemical attacks by-products from decaying food, etc.).
- The solubility is greater in dilute organic acids like lactic, acetic, and especially citric acids, all of which are present in the human diet. Thus it is important to minimize the exposure of the cement in the mouth by having minimum gaps at the margins of restorations.
Factors affecting solubility
- Powder-liquid ratio Thicker mixes show less solubility.
- Water content of liquid Any change in the water content is accompanied by increased solubility.
- Effect of moisture contamination Premature contact of the incompletely set cement with water results in the dissolution and leaching of the surface. Varnish application over the exposed cement margin is beneficial.
Film Thickness
The smaller the particle size, less is the film thickness. The thickness is lesser than the size of the particles because, during mixing the particles are crushed and dissolved. The thickness can also be reduced by applying pressure on the casting during seating.
Thermal Properties
Zinc phosphate cements are good thermal insulators and may be effective in reducing galvanic effects.
Adhesion Property
The primary retentive mechanism of zinc phosphate is micromechanical. The cemented restoration is held by mechanical interlocking of the set cement with surface roughness on the tooth and restoration.
Biological Properties
- pH of the cement The acidity is high at the time of insertion due to phosphoric acid. At the time of cementation, the pH is 2 (approx.). As time passes the acidity reduces. By the end of 24 hours, the pH is 5.5, which is still in the acidic range (neutral value is 7).
- Pulpal response The pulp response may be classified as moderate.
- Pulp protection A thickness of dentin as great as 1.5 mm can be penetrated by the acid of the cement. If dentin is not protected against infiltration of this acid, pulpal injury may occur, especially during the first few hours.
- Avoid thin mixes.
- Pulp protection should be carried out in deep cavities through the use of an intervening liner or base
- Zinc oxide eugenol
- Calcium hydroxide
- Cavity varnish
- Some patients are extremely sensitive to the acid. Cementation of a restoration such as a crown or FDP onto vital teeth can cause severe sensitivity or pain. Anesthesia should be used in these instances.
Optical Properties The set cement is opaque.
Optical Manipulation
- Spatula used Stainless steel.
- Mixing time 1 min. 15 seconds.
- Powder to liquid ratio 1.4 g/0.5 mL
A cool glass slab is used in order to delay the setting and allow more powder to be incorporated before the matrix formation occurs. The liquid should be dispensed just before mixing.
Procedure
The powder is added in small increments. Mixing is done with a stainless spatula using brisk circular motion. Each increment is mixed for 15 to 20 seconds. A large area is covered during mixing in order to dissipate the exothermic heat.
Maximum amount of powder should be incorporated in the liquid to ensure minimum solubility and maximum strength. Note: An appropriate consistency is attained by the addition of more powder to the liquid and not by allowing a thin mix to thicken.
Insertion
The crown should be seated immediately and held under pressure till set. The field of operation should be dry. Varnish is applied at the margins, where the cement is exposed.
Advantages And Disadvantages Of Zinc Phosphate
Advantages
- Long track record with proven reliability.
- Good compressive strength.
Disadvantages
- No chemical adhesion. Not indicated if the retention is poor.
- No anti-cariogenic property.
- Pulp irritation.
- Poor esthetics; cannot be used with translucent (all ceramic) restorations like crowns and veneers.
Copper Cement
Copper cements are basically modified zinc phosphate cements. Silver salts or copper oxide are sometimes added to the powders of the zinc phosphate cement to increase their ‘antibacterial’ properties.
Copper cements gradually fell out of favor because of their poor biological properties. It was highly acidic and the copper was considered toxic to the cell. This may have been due to the extremely high copper content (97%) in certain cement (Ames).
There has been a renewed interest in copper cements recently. New formulations have come out with lower copper content (2%). It is claimed that these new-generation copper Cement are safe and is especially recommended for indirect pulp capping and where there is active caries.
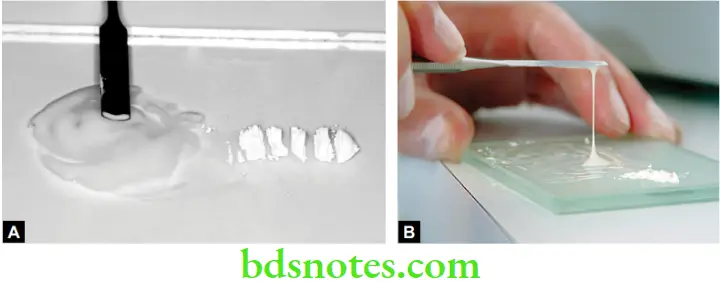
Copper Cement Applications
- Temporary fillings in children.
- Intermediate restorations.
- For retention of silver cap splints in oral surgery.
- Indirect pulp capping.
- As base beneath composite restorations.
Copper Cement Classification
- Classified according to the percentage of the copper oxide that is used as a replacement for the zinc oxide.
- Commercial examples Ames copper (discontinued), Doc’s best red and white copper kit
Copper Cement Composition
- Copper oxide (if cuprous oxide is used—cement is red, if cupric oxide is used, the cement is black)
- Zinc oxide
- The liquid used is clear phosphoric acid
Copper Cement Properties
- Biological properties: They have poor biological properties. Because its pH is 5.3, it is irritant to the pulp.
- They are bactericidal or bacteriostatic.
Copper Cement Manipulation
The chemistry of the copper cements is very similar to that of the zinc phosphate cements and they are manipulated in the same manner.
Zinc Polycarboxylate Cement
Canadian biochemist Smith developed the first polycarboxylate cement in 1968 by substituting the phosphoric acid of zinc phosphate cement with polyacrylic acid. Polycarboxylate became the first cement system developed with the potential for adhesion to tooth structure.

Zinc Polycarboxylate Cement Applications
- Primarily for luting permanent restorations.
- As bases and liners.
- Used in orthodontics for cementation of bands.
- Also used as root canal fillings in endodontics.
Zinc Polycarboxylate Cement Available As
- Powder and liquid in bottles
- Water settable cements
- As pre capsulated powder/liquid system
Zinc Polycarboxylate Cement Commercial Examples Poly F (Dentsply), Durelon and Durelon Maxicap (encapsulated) (3M ESPE), Carboco (Voco), Imibond P (Imicryl), Hy Bond polycarboxylate (Shofu).
Zinc Polycarboxylate Cement Water settable cements
In these cement the polyacid is freeze-dried and added to the cement powder. Water is used as the liquid. When the powder is mixed with water, the polyacrylic acid goes into the solution and the reaction proceeds as described for the conventional cements.
Zinc Polycarboxylate Cement Composition
Powder

Liquid
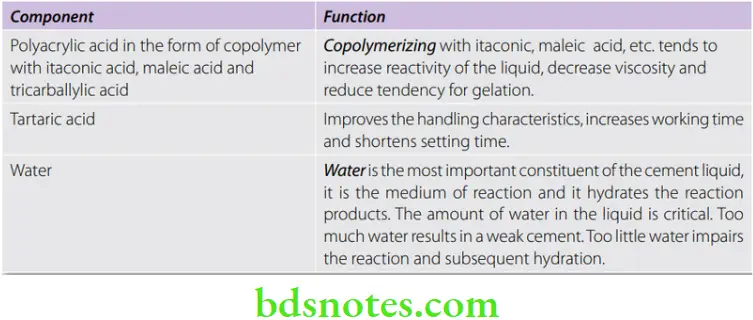
Aqueous solution of polyacrylic acid or copolymer of acrylic acid with other unsaturated carboxylic acids, i.e. itaconic, maleic, or tricarballylic acid.

Zinc Polycarboxylate Cement Manufacture
The powder mixture is sintered at high temperatures in order to reduce the reactivity and then ground into fine particles.
Zinc Polycarboxylate Cement Setting Reaction
- When the powder and liquid are mixed, the surface of powder particles are attacked by the acid, releasing zinc, magnesium and tin ions. These ions bind to the polymer chain via the carboxyl groups. They also react with carboxyl groups of adjacent polyacid chains to form cross-linked salts.
- Structure of set cement
- The hardened cement consists of an amorphous gel matrix of zinc polyacrylate in which unreacted powder particles are dispersed.
Properties
Mechanical properties
Compressive strength ISO requires a minimum compressive strength of 50 MPa for this cement . Polycarboxylate cement is inferior to zinc phosphate cement in this respect.Tensile strength 6.2 MPa. Its tensile strength is slightly higher than that of zinc phosphate cement.
The strength of the cement depends on
- Increase in P/L ratio increases strength.
- Molecular weight of polyacrylic acid also affects strength. A mix from a lower viscosity liquid is weaker.
Solubility and disintegration
- It tends to absorb water and is slightly more soluble (0.6% wt) than zinc phosphate. Thus the marginal dissolution is more when used for cementing. It is more soluble in organic acids like lactic acid. Low P/L ratio results in a significantly higher solubility and disintegration in the oral cavity.
Biocompatibility
Pulpal response is classified as mild. Despite the initial acidic nature of polycarboxylate cement, the pH of the liquid is 1.0–1.7 and that of freshly mixed cement is 3.0–4.0. After 24 hours, pH of the cement is 5.0-6.0.
They are less irritant than zinc phosphate cement because:
- The liquid is rapidly neutralized by the powder. The pH of polycarboxylate cement rises more rapidly than that of zinc phosphate.
- Penetration of polyacrylic acid into the dentinal tubules is less because of its higher molecular weight and larger size. The histological reactions are similar to zinc oxide eugenol cements but more reparative dentine is observed with polycarboxylate.
Adhesion
An outstanding characteristic of zinc polycarboxylate cement is that the cement bonds chemically with the tooth structure. The carboxyl group in the polymer molecules chelates with calcium in the tooth structure.
Bond strength to enamel is 3.4–13.1 MPa and to dentine 2.07 MPa.

Factors affecting bond
- A clean dry tooth surface improves bonding.
- If the inside surface of the metal crown is not clean, the cement cannot bond with the metal. So to improve the mechanical bond, the surface should be carefully abraded with a small stone or with airborne abrasives.
- The presence of saliva reduces bond strength.
- Unlike zinc phosphate cements, the adhesion is better to a smooth surface than to a rough surface.
- Does not adhere to gold or porcelain.
- Adhesion to stainless steel is excellent. Thus it is used in orthodontics.
Optical properties
- They are very opaque due to large quantities of unreacted zinc oxide.
Anticariogenic properties
- Some manufacturers have attempted to incorporate fluoride within the cement. However, the fluoride release is limited when compared to glass ionomer cement.
Thermal properties
- They are good thermal insulators.
Manipulation
Conditioning
- The tooth structure should be meticulously clean for proper bonding. To clean the surface, 10% polyacrylic acid solution followed by rinsing with water, or 1 to 3% hydrogen peroxide may be used. Then dry and isolate the tooth.
Proportioning
- 1.5 parts of powder to 1 part of liquid by weight.
Procedure
- The powder and liquid are taken on a cooled glass slab. The liquid is dispensed just prior to the mixing, otherwise its viscosity increases. The powder is incorporated into the liquid in bulk (90%) with a stiff cement spatula and remaining powder is added to adjust consistency. The mix appears quite thick, but this cement will flow readily into a thin film when seated under pressure.
Mixing Time And Setting Times
- Mixing time ranges from 30 to 40 seconds. Setting time can be from 7 to 9 minutes (The setting time can be increased by cooling the glass slab. It also depends on method of manufacture of powder and liquid).
Points to note
- The cement should be used while the surface is still glossy. Loss of lustre indicates that the setting reaction has progressed to an extent that proper wetting of the tooth surface by the mix is no longer possible. If the surface is not creamy and shiny and is matted and tends to form cobwebs, the mix should be discarded.
- After insertion the excess is not removed immediately as it passes through a rubbery stage, it tends to get lifted from the cavity. Remove excess cement only when it has hardened and breaks off.
- The powder may be cooled, but the liquid should not be cooled since the viscosity of the liquid increases.
Polycarboxylate cement adheres to instruments
- Use alcohol as release agent for mixing spatula.
- Instruments should be cleaned before the cement sets.
- Excess cement from the spatula can be chipped off. Any remaining material is removed by boiling in sodium hydroxide solution.
Advantages And Disadvantages
Advantages
- Comparatively less irritating to the pulp.
- Chemical bond to tooth structure.
Disadvantages
- Limited fluoride release when compared to GIC.
Zinc Oxide Eugenol Cement
These cements have been used extensively in dentistry since the 1890s. Depending on their use they vary widely in their properties. In general, they are cements of low strength. They are the least irritating of all dental cement and are known to have an obtundant (sedative) effect on exposed dentin.

To improve the strength many modified zinc oxide eugenol cements have been introduced, e.g. EBA—alumina modified and polymer—reinforced zinc oxide eugenol cements non eugenol zinc oxide cements are also available. They are suitable for patients sensitive to eugenol.
Classification (ISO 3107:2011)
Type 1—for temporary cementation
Type 2—for bases and temporary restorations
The previous version of this classification (ISO 3107:2004) * listing 4 classes, has been replaced by ISO 3107:2011, in which only 2 classes are described.
Type 1 cements are meant for short term luting (1 to 6 weeks—). They are used to cement provisional restorations for the period it takes to make the definitive restoration. Permanent restorations are also sometimes cemented for a short period for the patient to try it. They have low strength which allows easy removal of the restoration without damage to the restoration or the tooth.
Type 2 cements are used for the interim period (a few weeks to few months) when a tooth is undergoing treatment or until it is ready for a permanent restoration. They can also be used as bases under non-resin based permanent restorations.
Available As
- Powder and liquid
- Two paste system
Representative commercial names

Composition
Powder

Liquid

Setting Reaction
The setting reaction and microstructure are the same as that of the zinc oxide eugenol impression pastes.
In the first reaction hydrolysis of zinc oxide takes place. Water is essential for the reaction (dehydrated zinc oxide will not react with dehydrated eugenol).
⇒ \(\mathrm{ZnO}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Zn}(\mathrm{OH})_2\)
The reaction proceeds as a typical acid-base reaction.
⇒ \(\mathrm{Zn}(\mathrm{OH})_2+2 \mathrm{HE} \rightarrow \mathrm{ZnE}_2+2 \mathrm{H}_2 \mathrm{O}\)
The chelate formed is an amorphous gel that tends to crystallize imparting strength to the set mass.
Structure of set cement
Thus, the set cement consists of particles of zinc oxide embedded in a matrix of zinc eugenolate.

General Properties Of Zinc Oxide Eugenol Cements
Mechanical properties
- Compressive strength They are relatively weak cements. The strength depends on what it is used for, e.g. cements intended for temporary purposes like temporary restorations and cementation will have a lower strength. The compressive strength, therefore, ranges from a low of 5 to 55 MPa.
- Type 1 6 to 28 MPa (ISO—maximum of 35 MPa)
- Type 2 45 to 55 MPa (ISO—minimum of 5 MPa)
- Particle size affects the strength. In general the smaller the particle size, the stronger the cement. The strength can also be increased by reinforcing with alumina-EBA or polymers (see EBA and polymer modified ZOE cements).
- Tensile strength Ranges from 0.32 to 5.3 MPa.
- Modulus of elasticity (0.22 to 5.4 GPa) This is an important property for those cements intended for use as bases.
Thermal properties
- Thermal conductivity 3.98 [Cal. Sec-1 cm-2 (°C/cm)-1] × 10–4. Their thermal insulating properties are excellent and are approximately the same as for human dentin. The thermal conductivity of zinc oxide eugenol is in the range of insulators like cork and asbestos.
- Coefficient of thermal expansion 35 × 10–6/°C.
Solubility and disintegration
- The solubility of the set cement is highest among the cements (0.4 to 1.5 % wt). They disintegrate in oral fluids. This break down is due to hydrolysis of the zinc eugenolate matrix to form zinc hydroxide and eugenol. Solubility is reduced by increasing the powder/liquid (P/L) ratio.
Film thickness
- This property is important for those cements (Type I) used for luting of restorations. The film thickness of zinc oxide eugenol cements (ISO maximum of 25 µm) is generally higher than other cements.
Adhesion
- They do not adhere well to enamel or dentin. This is one reason why they are not often used for final cementation of crowns and other fixed dental prosthesis. The other reasons are low strength and high solubility.
Biological properties
- pH and effect on pulp (pH is 6.6 to 8.0): They are the least irritating of all cements. Pulpal response—classified as mild.
- Bacteriostatic and obtundent properties: They inhibit the growth of bacteria and have an anodyne or soothing effect (obtundent) on the pulp in deep cavities, reducing pain.
- Eugenol is irritating to skin and eyes. Repeated contact may cause allergic dermatitis.
Optical properties
- The set cement is opaque.
Material interactions
- Eugenol interferes with the hardening/and or cause softening of resin based restorations and are therefore contraindicated as a base under these restorations.
Manipulation
Powder/liquid system
Powder/liquid ratio 4:1 to 6:1 by weight.
- The bottles are shaken gently. Measured quantity of powder and liquid is dispensed onto a cool glass slab. The bulk of the powder is incorporated into the liquid and spatulated thoroughly in a circular motion with a stiff bladed stainless steel spatula. Zinc oxide eugenol exhibits pseudothickening. Although it appears to thicken early during spatulation. Further vigorous spatulation or stropping loosens the mix. Smaller increments are then added until the mix is complete.
- For temporary restorations a thick putty-like consistency is recommended.
- Oil of orange is used to clean eugenol cement from instruments.
Two paste system
- Equal lengths of each paste are dispersed and mixed until a uniform color is observed.
Setting time
- 4–10 minutes.
- ZOE cements set quicker in the mouth due to moisture and heat.
Factors affecting setting time
The complete reaction between zinc oxide and eugenol takes about 12 hours. This is too slow for clinical convenience.
- Manufacture The most active zinc oxide powders are those formed from zinc salts like zinc hydroxide and zinc carbonate by heating at 3,000 °C.
- Particle size Smaller zinc oxide particles set faster.
- Accelerators Alcohol, glacial acetic acid and water.
- Heat Cooling the glass slab, slows the reaction.
- Retarders The set can be retarded with glycol and glycerine
- Powder to liquid ratio Higher the ratio, faster the set.
Modified Zinc Oxide Eugenol Cements
These were introduced to improve some of the shortcomings of the regular unmodified zinc oxide eugenol. The modified ZOE cements are
- EBA-Alumina modified cements
- Polymer reinforced
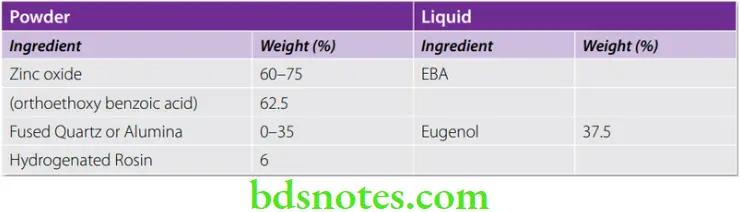
EBA-Alumina Modified Cements
These are modified ZOE cements. It is available as a white powder and a pinkish liquid. Its greater strength allows its use as an intermediate filling material and as a base. A part of the liquid is substituted by orthoethoxy benzoic acid. Alumina is added to the powder. These cements are increasing in popularity as a retrograde filling material because of the high cost of MTA.

Uses
- Long-term cementation.
- Temporary and intermediate restorations.
- Root end filling material.
Composition
Properties
Their properties are better than that of unmodified ZOE. They are more easier to handle and have improved carvability.
- Compressive strength is higher—55 to 60 MPa (8000 psi)
- Tensile strength—4.1 MPa (600 psi)
- Modulus of elasticity—2.5 GPa (0.36 psi × 106)
- Film thickness—25 µm
- Solubility and disintegration in water—0.05% wt. Despite their low solubility, these cements disintegrated and wore more quickly clinically when compared to the polymer modified zinc oxide cements.
- Effect on pulp—these cements are relatively mild to the pulp.
- Adhesion—these materials adhere well to tooth structure.
Manipulation
- A glass slab is recommended for EBA-alumina modified cements. After dispensing, the powder is incorporated into the liquid in bulk, kneaded for 30 seconds and then stropped for an additional 60 seconds with broad strokes of the spatula to obtain a creamy consistency. They have long working times.

Setting time
- 9.5 minutes.
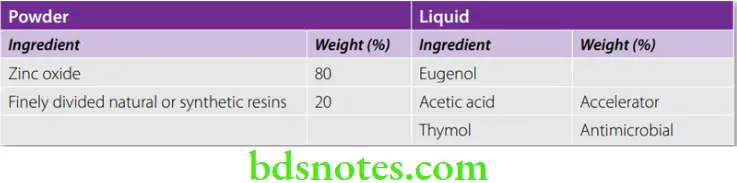
Polymer Reinforced Zinc Oxide Eugenol Cement
- The modifications take the form of resins added to the powder or the liquid. The aim is to improve the strength and reduce the solubility of the cement. Resin-modified cements are among the strongest of the zinc oxide eugenol based cements. Their high strength and low wear make them ideal intermediate restorative materials that can last as long as 1 year.
Uses
- Luting agent
- As base
- As temporary filling material
Available as 1. Powder and liquid. 2. Capsule for mechanical mixing.
Commercial Names IRM (Dentsply) and Kalzinol (DPI).
Composition
The zinc oxide powder is surface treated. The combination of surface treatment and polymer reinforcement results in good strength, improved abrasion resistance and toughness.
Setting Reaction
- The setting reaction is similar to zinc oxide eugenol cements. Acidic resins if present, may react with zinc oxide, strengthening the matrix.
Properties
These cements have improved mechanical properties.
- Compressive strength: 48 MPa (7000 psi)
- Tensile strength: 4.1 MPa (600 psi)
- Modulus of elasticity: 2.5 GPa
- Film thickness: 32 µm
- Solubility and disintegration: 0.03% wt
- Material interactions: Similar to ZOE these materials interfere with the hardening/ and or cause softening of composites and are therefore contraindicated as a base under resin based restorations.
- Pulp response: Classified as moderate which is similar to unmodified ZOE.
- Improved abrasion resistance and toughness.
Manipulation
- The proper powder/liquid is dispensed on a dry glass slab. 50 percent of the powder is mixed into the liquid and the remainder in small portions with vigorous spatulation or stropping. The mix will appear quite stiff, however continued stropping for an additional 5 to 10 seconds improves plasticity (known as shear thinning effect).
- After mixing, the plastic zinc oxide eugenol is swiped into the tooth cavity and condensed using a moist cotton pellet.
- Working time These cements have a long working time.
- Setting time 6 to 10 minutes. Heat and moisture in the mouth cause it to set faster than on the mixing pad.
Factors affecting setting time
- Low powder-liquid ratio increases setting time
- Moisture accelerates setting time.
- Cooling the glass slab slows the setting.
Other Zinc Oxide Eugenol Products
Endodontic Sealers
- Zinc oxide eugenol is very popular as an endodontic sealer. Two traditional formulations—Rickert’s formula and Grossman’s formula are very popular. Along with gutta-percha, these materials are used in endodontic therapy to seal the canals. Some materials are used as therapeutic sealers and are formulated with ingredients such as iodoform, paraformaldehyde or trioxymethylene which have therapeutic value. Others contain antibiotics such as tetracyclines and steroids as anti-inflammatory agents. Some formulations can also be used for pulp capping. Endodontic sealers also contain radiopaque materials such as barium sulphate, bismuth salts or silver powder. These products are discussed in further detail in a subsequent section.
Zinc Oxide/ Zinc Sulphate Cements
These are single component temporary filling materials. Their main advantage is their ease of placement.
Supplied As
- As putty in small tubes, syringes or plastic containers.
- Representative products Cavit (ESPE), Caviton (GC), Coltosol (Coltene).
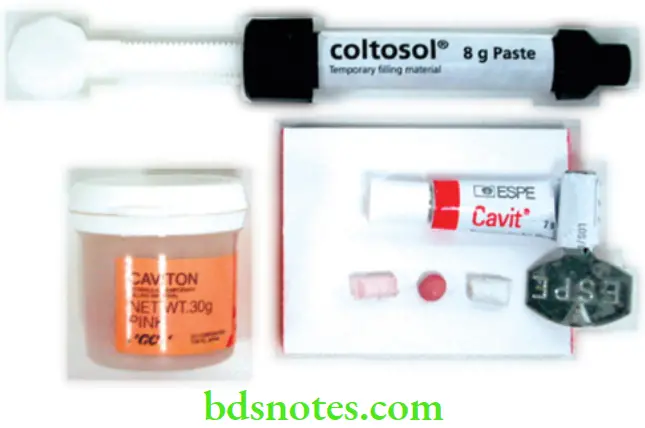
Use
- Short-term restorations after caries excavation, root canal therapy, etc.
Composition
- Zinc oxide 40–60 %
- Zinc sulphate-1-hydrate 1–20 %
- Calcium sulphate-hemihydrate 15–35 %
- Ethylene bis (oxyethylene) diacetate – 15–35 %
- Barium sulphate 0–20 %
- Poly (vinyl acetate)
- Diatomaceous earth
Setting Reaction
- The material sets by reacting with water which it absorbs from the mouth or from the cavity. The setting occurs slowly. It expands on setting.
Properties
- It may be white or pink colored putty-consistency material. It has good initial sealing. Since it expands on setting (up to 18%), the marginal seal is further improved. The seal gradually decreases with time as it disintegrates. Unfortunately, the strength is low and its life is short. The material should be used for not more than 1 to 2 weeks. It slowly disintegrates with time and is therefore not indicated for any longer term temporary restorations. The material is radiopaque. Short-term pain may be experienced because of dehydration of the cavity.
Manipulation
- The material is dispensed and inserted into the cavity using a cement carrier. The container should be closed immediately. It is condensed into the cavity using a plastic filling instrument (condenser). Since it sets by hydration, the cavity should not be fully dried before placing the material.
Setting time
- The surface hardens in about 20 to 30 minutes. Complete hardening takes place in 2 to 3 hours.
Glass Ionomer Cements
Glass ionomer cements are adhesive tooth-colored anticariogenic restorative materials which were originally used for restorations of eroded areas. Current glass ionomers have been modified to allow a wider application. These cements evolved from a general dissatisfaction with silicate cements. The first usable glass ionomer system was formulated in 1972 by Wilson and Kent and was known as ASPA. Subsequently great improvements were made and today these materials are very popular and widely used.
It was named glass ionomer because, the powder is a type of glass and the setting reaction and adhesive bonding to tooth structure is due to ionic bond. Unlike other restorative materials, this cement requires minimal cavity preparation as it bonds adhesively to tooth structure. Compared to composite resin they are less technique sensitive. Glass ionomer cement is often known as a biomimetic material, because of its similar mechanical properties to dentine. For this reason it is one of the most popular cements in dentistry.
Synonyms
- Poly (alkenoate) cement
- GIC (glass ionomer cement)
- ASPA (alumino silicate polyacrylic acid)
Application
- Anterior esthetic restorative material for Class 3 cavities.
- Restorative material for eroded areas and Class 5 restorations.
- As a luting agent for restorations and orthodontic brackets.
- As liners and bases.
- For core build up.
- To a limited extent as pit and fissure sealants.
- Intermediate restorative material.
- Atraumatic restorative treatment (ART) technique.
Glass ionomer cements are not recommended for Class 2 and Class 6 restorations, since they lack fracture toughness and are susceptible to wear.
Classification
The general ISO classification of cements apply to glass ionomer (ISO 9917-1:2007)*
- Luting
- Bases and liners
- Restorations
Difference between various types
The various types of GIC cements are chemically identical. They vary primarily in the powder/liquid ratio and particle size. The GICs used for luting have a lower powder/liquid ratio and a smaller particle size when compared to the restorative variety. These features enable the luting GIC to have a thinner film and better flow.
They may also be classified as
- Conventional GIC
- Resin-modified GIC
- Metal-modified GIC
Representative commercial products
Aquacem, Fuji 1 — Luting
Ketac bond — Bases and liners
Chem Fil, Fuji 2 — Restorations
Vitra bond — Light cure GIC
Available As
- Powder/liquid in bottles

- Preproportioned powder/liquid in capsules
- Light cure system
- Powder/distilled water (water settable type)
Composition
Powder
The powder is an acid-soluble calcium fluoroaluminosilicate glass. It is similar to that of silicate, but has a higher alumina-silica ratio. This increases its reactivity with liquid.

The fluoride component acts as a ‘ceramic flux’. Lanthanum, strontium, barium or zinc oxide additions provide radiopacity.
Liquid
Originally the liquid was a 50% aqueous solution of polyacrylic acid. It was very viscous and had a tendency to gel. Modern glass ionomer liquids are in the form of copolymers.
Water settable cements
- The polyacrylic acid copolymer is freeze dried and then added to the glass ionomer powder. The liquid is water or water with tartaric acid. An example of a water settable cement.
- When the powder is mixed with water, the polyacrylic acid powder goes into solution to form liquid acid. Then the chemical reaction takes place as in the conventional powder and liquid systems. These cements are known as water settable cements and they set faster than those with polyacrylic acid.

Manufacture
- The components are sintered at 1100 °C to 1500 °C. The glass is then ground to particle sizes ranging from 15 to 50 um.
Setting Reaction
- Leaching When the powder and liquid are mixed together, the acid attacks the glass particles. Thus calcium, aluminum, sodium and fluoride ions leach out into the aqueous medium.
- Calcium cross-links The initial set occurs when the calcium ions cross-links (binds) the polyacrylic acid chains. This forms a solid mass.
- Aluminum cross-links In the next phase, the aluminum also begins to cross-link with polyacrylic acid chains.
- Sodium and fluorine ions These ions do not take part in the cross-linking. Some of the sodium ions may replace the hydrogen ions in the carboxylic groups. The rest combine with fluorine to form sodium fluoride which is uniformly distributed within the cement.
- Hydration Water plays a very important role in the cement. Initially it serves as the medium. Later it slowly hydrates the matrix, adding to the strength of the cement (maturation process).
- Silica gel sheath The unreacted glass (powder) particle is sheathed (covered) by a silica gel. It is formed by the leaching of the ions (Ca²+, Al³+, Na+, F¯) from the outer portion of the glass particle.
Structure of set cement
- The set cement consists of agglomeration of unreacted powder particles surrounded by a silica gel sheath and embedded in an matrix of hydrated calcium and aluminum cross-linked polyacrylic gel.

Sensitivity to air and moisture
- Exposure of the cement to water before the hardening reaction is complete, leads to loss of cations and anions which form the matrix as they can be dissolved. Thus it is very important to protect the cement surface (by applying varnish, etc.) after it is placed in the mouth.
Properties
- Some of minimum requirements for the different types of GI cements.
Mechanical properties
Compressive strength Because of differences in the powder-liquid ratio GIC used for different applications show variations in their physical properties. Restorative GIC has a compressive strength of 150 MPa. The luting GIC has a lower compressive strength of about 85 MPa.
- Tensile strength Luting type – 6.2 MPa
- Restorative type – 6.6 MPa
Hardness (49 KHN) Less harder than silicates. The hardness is also far lower when compared to composites.
Fracture toughness A measure of energy required to produce fracture. Type II GIC’s are far inferior to composites in this respect.
Elastic modulus (7.3 GPa) It is a measure of their stiffness. The MOE is half that of zinc phosphate cement.
Wear resistance They are more susceptible to tooth brush abrasion and occlusal wear when compared to composites.
Solubility and disintegration
The initial solubility is high due to leaching of intermediate products. The complete setting reaction takes place in 24 hours; therefore, the cement should be protected from saliva in the mouth during this period. Glass ionomer cements are more resistant to attack by organic acids.
- Solubility in water for Luting type—1.25% wt.
- Solubility in water for Restorative type—0.4% wt.
Adhesion
- It adheres well to enamel and dentin. Shear bond strength ranges from 3–5 MPa.
- Mechanism of adhesion Glass ionomer bonds chemically to tooth structure. The exact mechanism has not been fully understood. The bonding is due to the reaction between the carboxyl groups of the polyacids and the calcium in the enamel and dentin. The bond to enamel is always higher than that to dentin, probably due to the greater inorganic content of enamel and its greater homogeneity.
Esthetics
- Esthetically they are inferior to silicates and composites. They lack translucency and have a rough surface texture. They may stain with time. The restorative GICs are available in different shades. The esthetics are sufficient for restoring cervical lesions and minor defects in nonesthetic zones. The luting cement is more opaque than the restorative cement.
Biocompatibility
- Pulpal response to GIC is classified as mild.
- Type 2 glass ionomers are relatively biocompatible. The pulpal reaction is greater than that from zinc oxide eugenol cements but less than that produced by zinc phosphate cement. Polyacids are relatively weak acids.
- The water settable cements show higher acidity. Luting type GIC is more acidic thanRestorative type because of the lower powder/liquid ratio. Occasionally sensitive patients show a painful response to GIC luting cement.
- Pulp protection In deep cavities, the smear layer should not be removed as it acts as a barrier to acid penetration. Deep areas are protected by a thin layer of calcium hydroxide cement.
Anticariogenic properties
- Type 2 glass ionomer releases fluoride in amounts comparable to silicate cements initially and continue to do so over an extended period of time.
- In addition, due to its adhesive effect they have the potential for reducing infiltration of oral fluids at the cement-tooth interface, thereby preventing secondary caries.
Manipulation
- Conditioning of tooth surface.
- Proper manipulation.
- Protection of cement during setting.
- Finishing.
Preparation Of Tooth Surface
- The tooth should be clean for effective adhesion of cement. The smear layer present after cavity preparation tends to block off the tooth surface and so should be removed to achieve adhesive bonding.
- This is achieved by
- Rubbing with a cotton pellet and pumice slurry
- Etching with 10% polyacrylic acid or 37% phosphoric acid.
- (The objective is to remove the smear layer but still leave the collagenous plug in place. The plug acts as a barrier to the penetration of acid from the cement).
- Conditioning This is achieved with 10% polyacrylic acid or 37% phosphoric acid for about 10 to 20 seconds. Next rinse with water for 20 seconds. Very deep areas of the preparation should be protected by a dab of calcium hydroxide.
- After conditioning and rinsing, the surface is dried but not desiccated. It should be kept free of saliva or blood as these will interfere with bonding. If contaminated the whole procedure is repeated.
Proportioning And Mixing
Powder/liquid ratio
- Powder/liquid ratio varies according to the type of GIC and intended use.
- Most manufacturers provide a plastic scoop which is useful for measuring. The manufacturers recommended ratio should be followed. Low P/L ratio reduces mechanical properties and increase the chances of cement degradation. Moisture contamination alters the acid-water balance.
- Spatula used Stiff plastic or metal spatula.
Mixing
- Manual mixing The powder bottle is tumbled gently. The powder and liquid is dispensed just prior to mixing. A nonabsorbent paper pad or a cool and dry glass slab may be used.
- The powder is divided into two or more increments. The first increment is incorporated rapidly into the mix with a stiff bladed spatula in about 5–10 seconds. The material should not be spread over a large area. Subsequent increments are incorporated and mixed using a swiping and folding technique. The material is collected and folded on to itself. Total mixing time should not exceed 30–40 seconds.
- A good mix should have a glossy surface. This indicates the presence of residual polyacid (which has not been used up in the setting reaction) and ensures proper bonding to the tooth. A mix with dull surface is discarded as it indicates prolonged mixing and reduces the adhesion.
- Mixing time 45 seconds.
- Insertion The mix is packed into the cavity without delay using a plastic filling instrument. If the mix loses its gloss or forms a skin it should be discarded.
- Mechanical mixing GIC supplied in capsule form containing preproportioned powder and liquid is mixed in an amalgam triturator. The capsule has a nozzle and so the mix can be injected directly into the cavity or crown.


Consistency after mixing
- This varies according to the type of GIC and its intended use. For example, restorative consistency differs from luting consistency. For luting the material should have sufficient flow to ensure complete seating. Care should be taken not to make it too fluid as it can reduce strength. For restorations, a thicker consistency is required to provide sufficient body for manipulation and placement into the cavity. In the ART (atraumatic restorative treatment) technique the material has a very heavy or putty like consistency for improved packability.
Advantages
- Better properties due to controlled P/L ratio.
- Less mixing time required.
- Convenient delivery system.
Disadvantages
- Cement quantity limited by the manufacturer.
- Shade selection is limited, colors cannot be blended.
Setting time
- Luting type — 7 minutes
- Restorative type — 4 to 5 minutes
Protection And Shaping Of Cement After Setting
Glass ionomer cement is sensitive to air and water during setting. It should be protected from moisture contamination as well as drying during setting and for a few days after setting. After placement into the cavity, a preshaped matrix may be applied to
- Protect the cement from the environment while setting.
- Provide maximum contour so that minimal finishing is required.
- Ensure adequate adaptation on to the walls of the cavity.
Protection Of Cement After Setting
The matrix is removed after complete set. Immediately after removal, the cement surface is again protected from drying with
- A special varnish supplied by manufacturer, or
- An unfilled light cured resin bonding agent, or
- Cocoa butter or petroleum jelly

This protects the cement from drying while the dentist proceeds with the finishing. Failure to protect the cement surface from contact with air results in a chalky or crazed surface.
The causes for chalky or crazed surface are
- Inadequate protection of freshly set cement (from air)
- Low powder/liquid ratio
- Improper manipulation
Finishing
- Excess material is trimmed from the margins. Hand instruments are preferred to rotary tools to avoid ditching. Further finishing if required is done after 24 hours.
- Before dismissing the patient, the restoration is again coated with the protective agent to protect the trimmed areas. Failure to protect the cement from saliva for the first 24 hours can weaken the cement.
Precautions
- If the liquid contains polyacids, it should not be placed in a refrigerator as it becomes very viscous.
- The restorations must be protected from drying at all times, even when other dental procedures are to be carried out later.
- The glass slab should not be below dew point, as moisture may condense on the slab and change the acid-water balance.
Packable Glass Ionomer For Posterior Restorations
A packable GIC with a dough like consistency is available as a cheaper alternative to compomers and composites for posterior restorations.
Indications for packable GIC
- Pediatric and geriatric restorations.
- Intermediate restorative material.
- Permanent restorative material in non-stress zones.
- As a core material.
Advantages
- Higher wear resistance than conventional GICs.
- Packable and pressable.
- Fluoride release.
- Simple to place (single step).
- Less technique sensitive
Atraumatic Restorative Dentistry (ART)
- In areas with no access to electricity or equipment, patients may be treated using the ART concept which involves hand excavation of caries. Since hand excavation is often incomplete, one has to rely on a materials that bonds adhesively to enamel and release fluoride in order to protect teeth under adverse conditions. The material of choice in this case is packable GIC.

Fissure Sealing (Special Applications)
- The traditional glass ionomer cement is somewhat viscous, which prevents penetration to the depth of the fissure. Thus the fissure orifice in general must exceed 100 um in width. Fissures or pits that are smaller are better treated with acid etching and light cured resin sealants. The use of glass ionomer in sealant therapy will increase as formulations are developed that are less viscous (e.g. light cured) and have good wear resistance.
Modified Glass Ionomers
Over the years glass ionomer has been modified by manufacturers in order to compensate for some of their deficiencies. This has resulted in new products. The modified glass ionomers are:
- Metal modified GIC
- Resin modified GIC
Metal Modified Glass Ionomer Cement
Metal-reinforced glass ionomer cements were first introduced in 1977 to improve the strength, fracture toughness and resistance to wear and yet maintain the potential for adhesion and anticariogenic property. The addition of silver-amalgam alloy powder to conventional materials also provided radiodensity. Subsequently, silver particles were sintered onto the glass and a new product called cermet was launched. These materials are currently considered old fashioned, as the conventional glass ionomer cements have comparable physical properties and far better esthetics.
Types
Two methods are employed
- Silver alloy admixed Spherical amalgam alloy powder is mixed with restorative type GIC powder.
- Cermet Silver particles are bonded to glass particles. This is done by sintering a mixture of the two powders at a high temperature (Ketac-Silver).
Uses
- Restoration of small Class 1 cavities as an alternative to amalgam or composite resins. They are particularly useful in young patients who are prone to caries.
- For core-build up of grossly destructed teeth.


Properties
Mechanical properties
- The strength of either type of metal modified cement (150 MPa) is not greatly improved over that of conventional cement.
- Diametral tensile strength of the cement is similar to conventional GIC.
- The fracture toughness of metal modified GIC is similar to that of conventional GIC.
- In the mouth both metal modified and conventional GIC appear to have similar wear rates.
From the above properties it is clear that there is no appreciable advantage of using metal modified GIC over conventional GIC. The clinical performance of cermet cements is considered to be inferior to other restorative materials, so much so that their use is now discouraged.
Anticariogenic property
- Both metal modified ionomers have anticariogenic capability due to leaching of fluoride. However, less fluoride is released from Cermet cement than restorative GIC, since the glass particle is metal coated. On the other hand the admixed cement releases more fluoride than restorative GIC. Here the metal filler particles are not bonded to the cement matrix and thus there are pathways for fluid exchange. This increases leaching of fluoride.
Esthetics
- These materials are gray in color because of metallic phases within them; therefore, they are unsuitable for use in anterior teeth.
Resin – Modified Glass Ionomer
These are relatively new materials having various names like compomer, resin-ionomers, RMGI (resin-modified glass ionomer), light cured GIC, dual cure GIC, tricure GIC, reinforced GIC, hybrid ionomers, etc. These materials were developed to overcome some of the drawbacks of conventional GIC like
- Moisture sensitivity
- Low initial strength
- Fixed working times.
Classification
Depending on which is the predominant component. These materials may be classified as (McClean et al).

- Resin-modified glass ionomer cement (RMGI), e.g. Fuji II LC, Vitremer, Photac Fil, etc.
- Compomers or polyacid-modified composites (PMC), e.g. Dyract Variglass VLC (this category will be discussed subsequently under the heading compomers).
Uses
- Restoration of Class 1, 3 or 5 cavities.
- Bases and liners.
- As adhesives for orthodontic brackets.
- Cementation of crowns and FDPs.
- Repair of damaged amalgam cores or cusps.
- Retrograde root filling.
Note Uses vary according to brand.
Supplied As
They are supplied as
- Chemical cure (acid-base setting reaction of the glass ionomer portion).
- Dual cure (combines acid-base setting reaction of the GIC portion and light curing of the resin portion).
- Tricure (combines acid-base setting reaction, chemical and light cured polymerization of the resin portion).
All of them are usually supplied as powder and liquid. The light cured type is supplied in dark shaded bottles (for light protection).
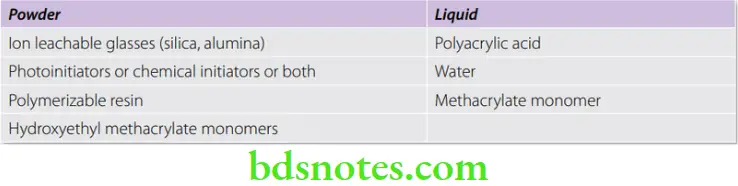
Composition
Since these are combination materials, they contain components of both resin and glass ionomer. However, their proportions vary.
Setting Reaction
- Setting includes both polymerization and acid-base reaction. The initial setting occurs by polymerization of the methacrylate groups giving it a high early strength. Polymerization may be light cured or chemical cured depending on the type of cement. Subsequently the acid-base reaction sets it thereby completing the setting reaction and giving the cement its final strength.
Manipulation
- RMGI is mixed and applied after conditioning the tooth with polyacrylic acid (10–25%). The powder and liquid is mixed according to the manufacturer’s instruction. Light cured RMGI is cured by exposure to blue light (which is used for curing composite).
Properties
Strength
- The compressive strength is slightly lower (105 MPa) when compared to conventional GIC. The diametral tensile strength is however greater (20 MPa). They have a greater fracture toughness because of the greater resilience of the resin component.
Hardness
- The hardness (40 KHN) is comparable to that of conventional GIC.
Adhesion
- The bonding mechanism to tooth structure is similar to that of conventional GIC. Micromechanical retention also plays a role in the bonding process. These materials bond better to composite resins than conventional GIC. This may be because of the presence of residual unreacted monomers within the RMGI.
Microleakage
- These materials have a greater amount of microleakage when compared to GIC. This may be partly due to the polymerization shrinkage and partly due to the reduced wetting of the tooth by the cement.
Anticariogenicity
- These materials have a significant anticariogenic effect because of the fluoride release. Some tests indicate fluoride release may be equivalent to that of conventional GIC.
Pulpal response
- The pulpal response to the cement is mild (similar to conventional GIC).
Esthetics
- They are more translucent and therefore more esthetic than conventional GIC. This is due to the closeness of the refractive indices of the powder and the monomer in the liquid.
Calcium Hydroxide Cement
Calcium hydroxide is a relatively weak cement commonly employed as direct or indirect pulp capping agents. Due to their alkaline nature they also serve as a protective barrier against irritants from certain restorations.
A light cured calcium hydroxide base material and a calcium hydroxide root canal sealing paste is also available.
Applications
- For direct and indirect pulp capping.
- As low strength bases beneath restorations for pulp protection.
- Apexification procedure in young permanent teeth where root formation is incomplete.
Available As
- Two paste system containing base and catalyst pastes in soft tubes
- Light cured system
- Single paste in syringe form
- Powder form (mixed with distilled water)
- Some representative commercial products
- Regular set—Dycal (Dentsply), Calcidor (Dorident), Recal (PSP), Hydrox (Bosworth)
- Light cured—Septocal LC (Septodont) and Calcimol LC (VOCO).

Composition
Base paste

Catalyst paste
Setting Reaction
- Calcium hydroxide reacts with the l-methyl trimethylene disalicylate ester to form a chelate viz. amorphous calcium disalicylate. Zinc oxide also takes part in the reaction.
Ca (OH)2+ l-methyl trimethylene disalicylate → calcium disalicylate
Properties
- Calcium hydroxide cements have poor mechanical properties. However, they are better than zinc oxide eugenol.
Mechanical properties
- Compressive strength (10-27 MPa after 24 hours). It has a low compressive strength. The strength continues to increase with time.
- Tensile strength (1.0 MPa) is low.
- Modulus of elasticity (0.37 GPa/m2). The low elastic modulus limits their use to areas not critical to the support of the restoration.
Thermal properties
- If used in sufficiently thick layers they provide some thermal insulation. However, a thickness greater than 0.5 mm is not recommended. Thermal protection should be provided with a separate base.
Solubility and disintegration
- The solubility in water is high (0.4–7.8%). Some solubility of the calcium hydroxide cement is necessary to achieve its therapeutic properties. Solubility is higher when exposed to phosphoric acid and ether. So care should be taken during acid etching and during application of varnish in the presence of this cement.
Biological properties
- Effect on pulp: The cement is alkaline in nature. The high pH is due to the presence of free Ca(OH)2 in the set cement. The pH ranges from 9.2 to 11.7.
- Formation of secondary dentin: The high alkalinity and its consequent antibacterial and protein lysing effect helps in the formation of reparative dentin.
Adhesion
- The material is sensitive to moisture and does not adhere in the presence of blood, water or saliva. The adhesive bond is weak.
Manipulation
- Equal lengths of the two pastes are dispensed on a paper and mixed to a uniform color. The material is carried and applied using a calcium hydroxide carrier or applicator (a ball-ended instrument). It is applied to deep areas of the cavity or directly over mildly exposed pulp (contraindicated if there is active bleeding).
Setting Time
- Ranges from 2.5 to 5.5 minutes.
- Factors affecting setting time The reaction is greatly accelerated by moisture and accelerators. It therefore sets faster in the mouth.
Light Activated Calcium Hydroxide Cement
- Light activated calcium hydroxide cements are available. It consists of calcium hydroxide and barium sulphate dispersed in a urethane dimethacrylate resin. It also contains HEMA and polymerization activators. Some contain fluoride.
- Light activated cements have a long working time and is less brittle than the conventional two paste system. They are radiopaque. They are supplied in syringe form and is expressed directly on to the tooth through a replaceable nozzle. Examples are Septocal LC (Septodont) and Calcimol LC (VOCO).

Calcium Hydroxide Root Canal Sealing Pastes
Root canal sealers containing calcium hydroxide are available. These are similar to the ones used for pulp capping but contain increased amount of retarders in order to extend the working time while they are being manipulated in the warm environment of the root canal. They are also radiopaque.
Commercial names Sealapex (Kerr), Pulpdent, etc.
Their advantages are
- Effective antibacterial properties without irritation.
- They stimulate hard tissue repair in the apical foramen.
Resin Cements
Resin cements based on methyl methacrylate have been available since 1952 for cementation of inlays, crowns and other appliances. Development of resin cements came naturally with the development of composites resins. They are essentially low viscosity flowable composites. These cements are known for their high esthetics and high bond strengths. They were widely used for the cementation of orthodontic brackets and resin-bonded restorations. The development of esthetic all-ceramic restorations led to a renewed interest in an esthetic bonding system which complemented the esthetics of the restoration. The color of the underlying cement can influence the esthetics in translucent restorations. The resin cement also improves the esthetics at the margins of the restoration. According to some studies resin cements reduce fractures of all-ceramic restorations. Thus, they are popular for the cementation of all-porcelain restorations.
Applications
- For bonding of orthodontic brackets to acid-etched enamel.
- Cementation of porcelain veneers and inlays.
- Cementation of all-porcelain crowns and FDPs.
- Cementation of etched cast restorations.
Classification
Based on curing system
- Chemical cure
- Light cure
- Dual cure
Chemically activated resins can be used for all types of restorations.
Light activated resins cannot be used in all situations because of problems of light penetration. Thus their use is limited to thin ceramic restorations which allows some passage of light, composite restorations like inlays, ceramic or plastic orthodontic brackets, etc.
Dual cure resins are used when the material being bonded allows some degree of light penetration, e.g. ceramic crown, brackets, inlays, etc. The resin around the margins are cured using light to initiate setting. The portions where light cannot penetrate cure subsequently by chemical reaction.
Supplied As
They are supplied in syringes
- Chemical cured
- Two paste system containing base and accelerator
- Single paste system with activator in the bonding liquid
- Light cured: Single paste system.
Most systems also include a bonding agent and etchant.
Representative Commercial Names Panavia F, Infinity, ResiLute (Pulpdent), Transbond XT (3M), Maxcem Elite (Kerr), Variolink Esthetic (Ivoclar) (Fig. 8.29A), etc.

Composition
- The resin cements have a composition similar to that of modern composites (refer chapter on composites). The filler content has to be lowered and diluent monomers are added to adjust the viscosity. Some contain fluoride (e.g. Panavia F).
- To promote adhesion to enamel and dentin, organophosphates (MDP), HEMA and 4 META are used as bonding agent.
Polymerization
- Chemically by peroxide-amine system
- Or by light activation
- Or by both chemical and light activation (dual cure).
Polymerization mechanisms are similar to those of resin-based composites.
Properties
Compressive strength : 180 MPa (26000 Psi)
Tensile strength : 30 MPa (4000 Psi)
Film thickness : 10–25 µm
Biological properties : Irritating to the pulp. Pulp protection with calcium hydroxide or GIC liner is necessary for areas close to the pulp.
Solubility : Insoluble in oral fluids.
Polymerization shrinkage : Is high
Adhesion properties : They do not adhere to tooth structure, which may lead to microleakage if used without etching and bonding.
Bond strength to enamel : 7.4 MPa (1070 Psi). Bond strength to enamel is usually strong. Failure most often occurs at the metal-resin interphase.
Manipulation And Technical Considerations
Like composites, resin cements are technique sensitive. Improper procedure can lead to poor bond strength and failure. The following processes are involved.
- Etching the restoration
- Etching the tooth surface
- Bonding and curing
- Removal of excess cement
Etching The Restoration
- Etching metal The metal surface can be etched or roughened by blasting with 30–50 µm alumina to improve retention. Etching is usually more effective. The process is carried out in a electrolytic bath containing an acid like sulfuric acid—also known as electrochemical etching. The non-bonding surface is protected with wax. Silica coating can also be used to improve bonding.
- Etching porcelain Ceramic is a highly inert material and is immune to attack by most acids. However, it can be etched by using hydrofluoric acid (refer chapter on ceramics). The esthetic surfaces are protected with a coating of wax.
- Orthodontic brackets In the case of orthodontic brackets, a fine mesh on the bonding side of the bracket helps to improve its retention. The cement flows into the mesh and locks to provide good mechanical retention. Coating with organosilane also improves bond strength.
Etching The Tooth Surface
- The tooth surface is etched with phosphoric acid (similar to procedure described in restorative resins). This is followed by an application of bonding agent.
Bonding And Curing
Chemically activated systems
- Two paste systems The two components are combined by mixing on a paper pad. Mixing time is 20–30 seconds.
- Single paste system with activator in bonding agent In some systems, the activator is present in the bonding agent. The bonding agent is painted on to the etched tooth surface as well as on to the restoration. Setting occurs when the cement on the restoration contacts the bonding agent on the tooth.
Dual cure system
- The two components are mixed and light cured.
- Time of exposure should never be less than 40 seconds.
- Light curing gives high initial strength.
- Light curing polymerizes the exposed cement at the margins of the restoration which is affected by air inhibition.
Removal Of Excess Cement
- Excess cement removal is critical. Removal of excess cement can sometimes be very difficult because of the high strength of the material. Therefore, removal of the excess cement should be attempted soon after seating before the material has fully hardened. Some manufacturers recommend a partial light cure to facilitate removal followed by completion of curing.
Compomer (Polyacid – Modified Composite Resins)
Shortly after the introduction of RM GICs, ‘compomers’ were introduced to the market. They were marketed as a new class of dental materials that would provide the combined benefits of composites (the ‘comp’ in their name) and glass ionomers (‘omer’). These materials had the fluoride release features of GIC with the durability of composite. Based on their structure and properties, these materials belong to the class of dental composites. Often they have been erroneously referred to as ‘hybrid glass ionomers’, ‘light-cured GICs’ or ‘resin-modified glass ionomers’. The proposed nomenclature for these materials is polyacid-modified composite resins, a nomenclature that is widely used in the literature.
Applications
- Restorative materials in pedodontics.
- Restorative material in nonstress bearing areas.
- Class 5 lesions.
- Bases.
- Luting (permacem).
Their applicability as orthodontic adhesives, amalgam bonding systems and veterinary restorative materials has also been reported.
Supplied As
These materials are sensitive to moisture. They are usually supplied as
- Light cured single paste in moisture proof packets (Dyract, Compoglass)
- Powder/liquid (Principle)
- Two paste static mixing system (PermaCem).
Commercial names Restorative—Dyract (Dentsply), Compoglass (Ivoclar).
Luting —Permacem, Principle (Dentsply), etc.
Composition
These materials have two main constituents: dimethacrylate monomer(s) with two carboxylic groups present in their structure and a filler that is similar to the ion-leachable glass present in GICs. The ratio of carboxylic groups to backbone carbon atoms is approximately 1:8. There is no water in the composition of these materials and the ion-leachable glass is partially silanized to ensure some bonding with the matrix.
- Single component system – Silicate glass, sodium fluoride, and polyacid modified monomer, photoinitiator.
- Double component system Powder – Glass fillers, accelerators, initiator, TiO2
- Liquid – Acrylic monomers, photoinitiator, water, carboxylic acid dimethacrylate.
Setting Reaction
- The initial set is via a free radical polymerization reaction activated by light. Subsequently water from saliva is absorbed by the cement and an acid-base reaction sets in between the carboxylic groups and areas of filler not contaminated by the silane coupling agents. It is this reaction which releases fluoride.
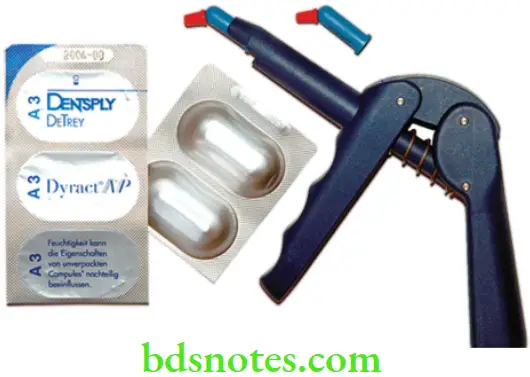


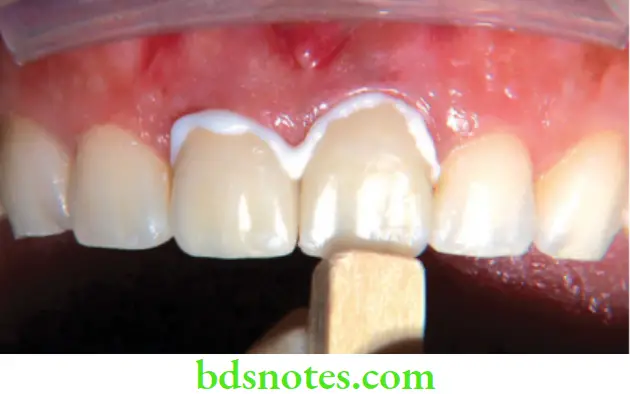
Bonding And Curing
Bonding and curing mechanisms are similar to the resin luting cements.
- Light cured
- Chemically cured
- Dual cured
Etching and bonding are similar to the resin luting cements. Some current materials are self etching and bonding. No additional etching and bonding are required in these materials.
Manipulation
- For the single component system the tooth is etched and bonding agent applied. The material is injected into the cavity and cured by light.
- For the powder/liquid system the powder and liquid is dispensed and mixed according to the manufacturer’s instruction for 30 seconds.
- For the static mixing system, the material comes out mixed when it is extruded through the spirals in the mixing tips.
Properties
- Considering the low volume fraction filler and the incomplete silanization of the filler, it could be postulated that they are inferior to composites. Both in vitro and in vivo investigations have confirmed this expectation. Lower flexural strength, modulus of elasticity, compressive strength, flexural strength fracture toughness and hardness, along with significantly higher wear rates compared to clinically proven hybrid composites, have been reported for these materials. Their clinical performance received mixed reviews during in vivo clinical trials.
Fluoride release
- Though these materials release fluoride they have significantly lower levels of fluoride release than GICs. Although low, the level of fluoride release has been reported to last at least 300 days.
Adhesion
- Unlike glass ionomer they do not have the ability to bond to hard tooth tissues. Like composites acid etching and use of bond agents are necessary.
Biocompatibility
- With the exception of concerns about the release of HEMA from these materials, no other biocompatibility issues have been associated with their usage.
Advantages And Disadvantages
- The prime advantage of these materials are their fluoride release anticariogenic potential. The disadvantage is their lack of adhesion. Thus bonding agents are required which increase in the number of steps and time required for placement.
- Constant reformulations of these types of materials may eventually make them comparable or even superior to existing composites, but as long as they do not set via an acid-base reaction and do not bond to hard-tooth tissues, they cannot and should not be classified with GICs.

Leave a Reply