Messenger RNA Coding RNA
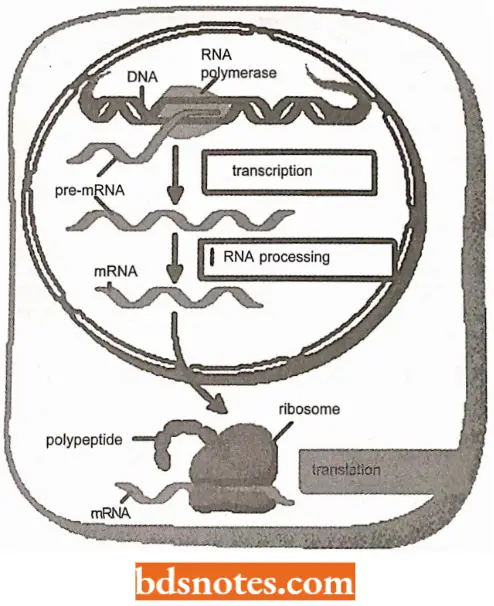
Messenger RNA (mRNA) acts as the intermediate molecule between the gene and the polypeptide translation product. Its existence was postulated by Crick and his associates during the 1950s.
- Among the indirect evidence for mRNA at that time was the knowledge that in eukaryotes the genes reside on the chromosomes in the nucleus, whereas protein synthesis occurs in ribosomes in the cytoplasm.
- The physical separation of genes and ribosomes means that some sort of messenger molecule must carry the biological information from the nucleus to the cytoplasm.
- In bacteria, the physical separation of DNA and ribosomes is less distinct but a messenger molecule is still necessary.
- Evidence that this messenger is RNA came first from Elliot Volkin and Lazarus Astrachan in 1956, but more convincingly from Sol Spiegelman and Benjamin D. Hall in 1961.
- Both groups demonstrated that after infection of a culture of bacteria with a bacteriophage, the new RNA that is synthesized is related in sequence to the phage DNA, suggesting that the phage genes are copied into RNA before synthesis of the phage proteins occurs.
- Shortly afterward two independent groups Brenner, Francois Jacob, and Mathew Meselson, who did the crucial experiment at the California Institute of Technology) and James Watson’s group at Harvard- directly identified mRNA molecules in E. coli cells.
Life Span Of mRNA
Messenger RNA molecules are not generally long-lived in the cell, i.e., most of them are unstable.
- Most bacterial mRNAs have a half-life of only a few minutes and so are turned over very rapidly.
- In eukaryotic cells, mRNA molecules have longer half-lives (for example., 6 hours for many mammalian mRNAs) but are still subject to turnover.
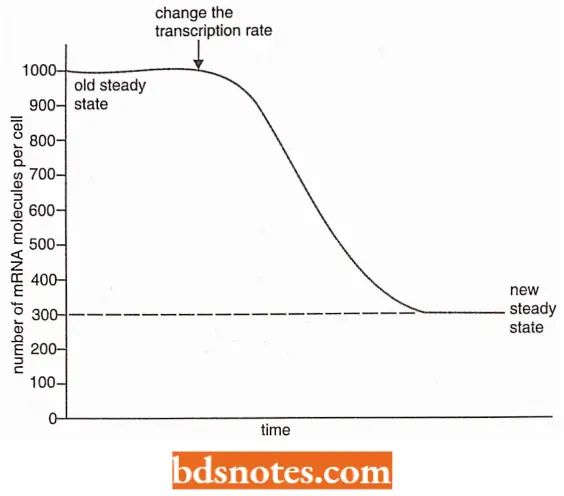
- Turnover of mRNA is important because it means that the absolute amount of a particular mRNA in the cell can be controlled by adjusting the rate at which the relevant gene is transcribed.
- If the transcription rate for the gene decreases then the level of the mRNA in the cell also decreases until a new steady state is reached.
- In fact, in both bacteria and eukaryotes mRNAs are known to be resistant to cytoplasmic ribonuclease enzymes and survive for long periods.
- For example, mRNA with a lifetime of six hours has been detected in the bacterium Bacillus cents at a time when the cells are induced to become long-lived spores.
- Likewise, in differentiating eukaryotic cells mRNAs with a lifetime of days have been detected.
- For example, in the immature red blood cells (reticulocytes) of mammals the mRNA is synthesized originally by the nucleus in early stages and expelled to the cytoplasm.
- In later stages, the nuclei of maturing reticulocytes degenerate but the mRNA exists for up to 2 days for prolonged utilization in the synthesis of globin protein of hemoglobin.
- Further, in extreme cases, such as in the state of dormancy adopted by many animal eggs and plant sips, M, mRNA is maintained in a stable form for months or even years.
Self-Splicing And Ribozyimes
Sidney Altman in 1981 showed that RNA can have catalytic properties. In 1982, Thomas Cech and Sidney Altman discovered self-splicing by RNA; both were awarded the 1989 Nobel Prize in Chemistry.
- Working with an intron in the 35S ribosomal RNA precursor in the single-cell eukaryote (ciliated protozoan) Tetrahymena thermophile.
- Cech and his colleagues found that they could induce intron removal in vitro with no proteins present. A guanine-containing nucleotide (GMP; GDP or GTP) had to be present.
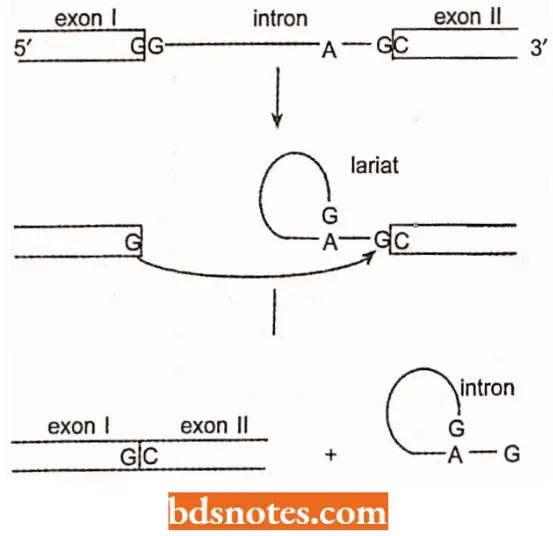
- The diagram depicts how self-splicing occurs. The intron acts as an enzyme; we call an RNA with catalytic or enzymatic properties a ribozyme.
- During self-splicing, the U-A bond at the left (5′) side of the intron is transferred to the GTP.
- The U that is now unbounded displaces the G at the right (3′) side of the intron, reconnecting the RNA with a U-U connection and releasing the intron.
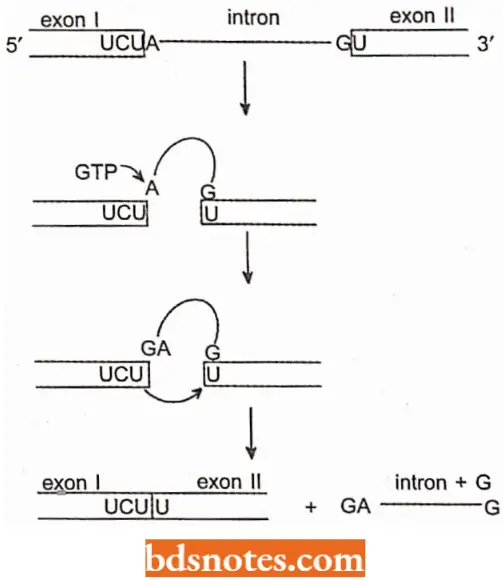
- Since all bonds are reversible transfers (transesterifications) rather than new bonds, no external source of energy is required. Self-splicing introns of this type are called group I introns.
- Although the first enzymatic activity of the ribozyme is its removal, its secondary structure after removal gives it the ability to further catalyze reactions.
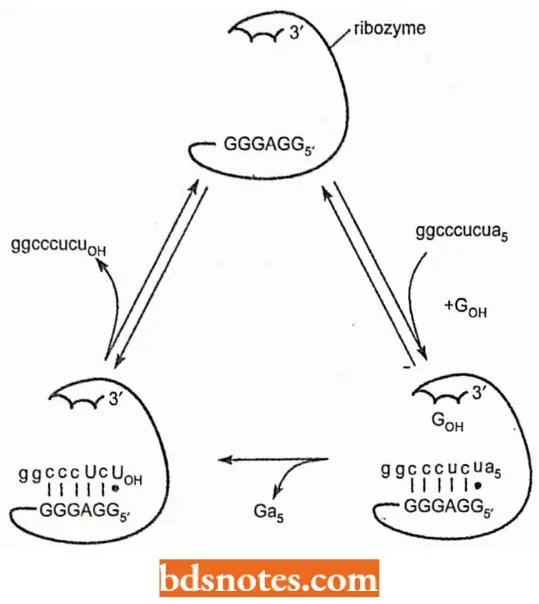
- The reactions that ribozymes catalyze are transesterifications and the hydrolysis reaction of splitting an RNA molecule into two parts.
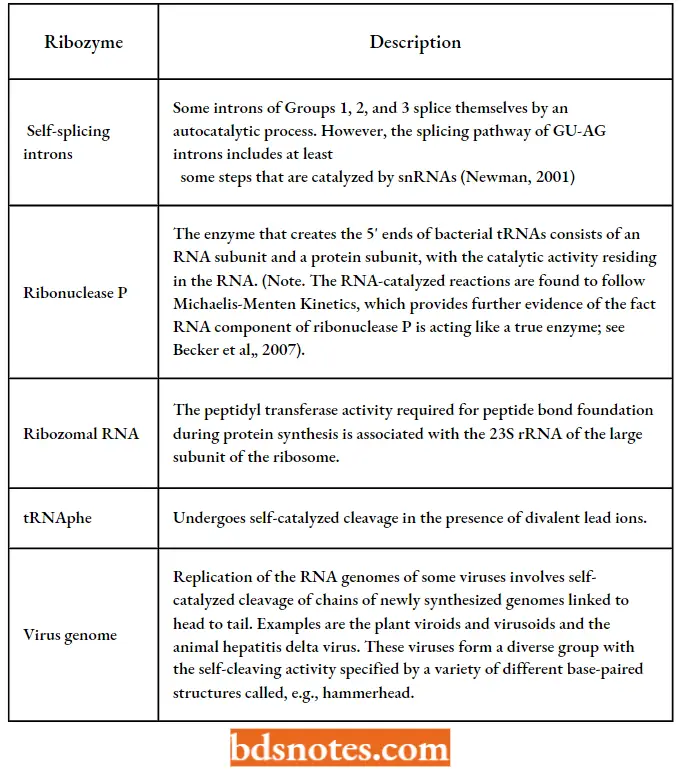
- Ribozymes can also perform other functions, including peptide bond formation. Currently, at least seven different classes of ribozymes are known, based on their enzymatic properties.
- A ribozyme that can split other RNAs and that occurs in small plant pathogens (viruses) is called a hammerhead ribozyme because of its shape.
- Self-splicing has also been found in genes in the mitochondria of yeast.
- These introns are referred to as group II introns because they use a different mechanism of splicing that does not require an external nucleotide.
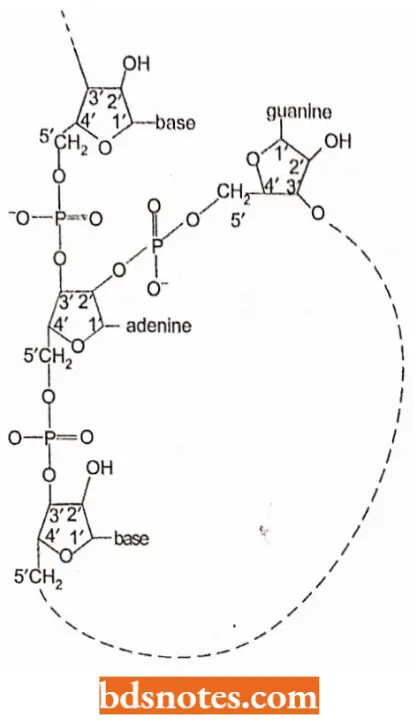
- Instead, the first bond is transferred within the intron to an adenosine, forming a lariat structure.
- For the lariat to form, the ribose of the adenosine must make three phosphodiester bonds.
Examples Of Ribozymes:
Structure of Ribozymes: There appear to be at least two kinds of ribozymes.
- Some ribozymes have folded structures and catalyze reactions on themselves, a process called intramolecular catalysis.
- Other ribozymes act on other molecules, without themselves being changed, a process called intermolecular catalysis.
- The ribozyme consists of a catalytic core made up of two domains,-each one comprising two of the base-paired regions, with the splice sites brought into proximity by an interaction between two other parts of the secondary structure.
- Although this RNA structure is sufficient for splicing, it is possible that with some introns the stability of the ribozyme is enhanced by non-catalytic protein factors that bind to it.
- For example, Group 1 introns in organelle genes, many of these containing an ORF coding for a protein called a maturase that appears to play a role in splicing.
Functions Of Ribozymes
Those ribozymes that are known today carry out three types of biochemical reactions:
- Self-cleavage: as displayed by the self-splicing group 1, 2, and 3 introns and by some virus genomes.
- Cleavage of other RNAs: as carried out by RNase P (discovered by Altman et al, 1983)
- Synthesis of peptide bonds: by the rRNA component of the ribosome (discovered by Harry Noller and his colleagues in 1992).
In the test tube, synthetic RNA molecules have been shown to carry out other biologically relevant reactions, such as;
- Synthesis of ribonucleotides (Unran and Bartel, 1998).
- Synthesis and copying of RNA molecules (Exland and Bartel, 1996: Johnston et al, 2001)
- Transfer of an RNA-bound amino acid forming a dipeptide, in a manner analogous to the role of tRNA in protein synthesis (Lohse and Szostak, 1996).
The discovery of these catalytic properties solved the polynucleotide-polypeptide dilemma by showing that the first biochemical systems could have been centered entirely on RNA (Bartel and Unran, 1990).
Role of Ribozymes in the Origin of Life: Ideas about the RNA world during chemical evolution and the origin of life have taken shape in recent years (Robertson and Ellington, 1998).
- Biologists now envisage that RNA molecules initially replicated slowly and haphazardly simply by acting as templates for binding of complementary nucleotides which polymerized spontaneously.
- This process would have been very accurate so a variety of RNA sequences would have been generated, eventually leading to one or more with incipient ribozyme properties that were able to direct their own, more accurate self-replication.
- It is possible that a form of natural selection operated so that the most efficient replicating systems began to predominate.
- Greater accuracy in replication would have enabled RNAs to increase in length without losing their sequence specificity, providing the potential for more sophisticated catalytic properties,
- Possibly culminating in structures as complex as present-day Group-J introns (for example. Tetrahymena tRNA intron) and ribosomal RNAs.
Chances Of Errors In Gene Splicing
Certain topological problems might pose chances of errors in gene splicing.
- The following two types of errors can commonly occur during the splicing of a gene:
- The first of these is the substantial distance that might lie between splice sites, possibly a few tens of kb, representing 100nm or more if the mRNA is in the form of a linear chain.
- A means is therefore needed to bring the splice sites into proximity.
- The second topological problem concerns the selection of the correct splice site.
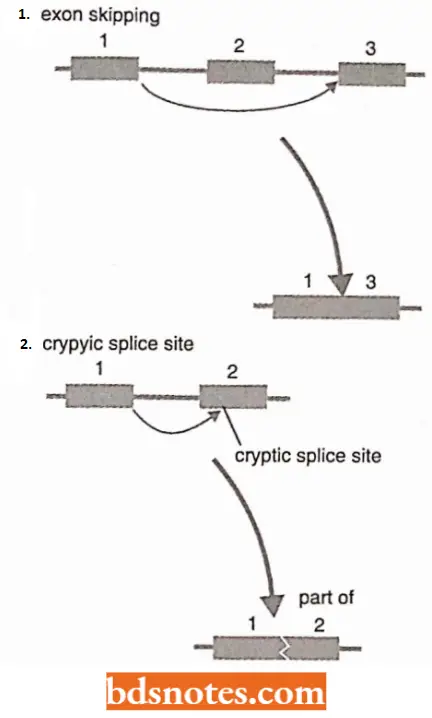
- All splice sites are similar, so if a pre-mRNA contains two or more introns then there is the possibility that the wrong splice sites could be joined, resulting in exon skipping, i.e., the loss of an exon from the mature mRNA.
- Equally unfortunate would be a selection of a cryptic splice site, a site within an intron or exon that has sequence similarity with consensus motifs of real splice sites.
- Cryptic sites are present in most pre-mRNAs and must be ignored by the splicing apparatus.

Leave a Reply