Chemical Nature Of The Genetic Materials Introduction
DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), the principal genetic materials of living organisms, are chemically called nucleic acids and are complex molecules larger than most proteins and contain carbon, oxygen, hydrogen, nitrogen, and phosphorus.
Historical
In 1869, Friedrich Miescher, a 22-year-old Swiss physician (working in the laboratory of Felix Hoppe-Seyler in Tubingen) had isolated from pus cells obtained from discarded bandages in the Franko-Prussian War, and from salmon sperm, a previously identified macromolecular substance, to which he gave the name nuclein.
- Although he was unaware of the structure and function of nuclein, he submitted his findings for publication. The editor who received the paper was doubtful about some aspects of Miescher’s report and delayed publication for two years while he tried repeating some of the more questionable aspects of Miescher’s work.
- Finally, in 1871, Miescher’s report was published, but it made little immediate impact. He continued his careful work up to his death in 1895, recognizing (with the help of his student Richard Altmann in 1889) that nuclein was of higher molecular weight and was associated in some way with a basic protein, to which he gave the name protamine.
- In 1880, Emil Fischer identified pyrimidines and purines. The biochemist, Albrecht Kossel identified the constituent nitrogenous bases of nuclein as well as its 5-carbon sugar and phosphoric acid.
- It was Altmann who first suggested, in 1899, the use of the term nucleic acid to describe phosphorus-containing nuclein. Kossel was awarded the 1910 Nobel Prize for demonstrating the presence of two pyrimidines (cytosine and thymine) and two purines (adenine and guanine) in nucleic acids.
- Kossel’s work and later investigations of Ascoli, Levine, and Jones during the first quarter of the 1900s disclosed the two kinds of nucleic acids, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The development of DNA-specific staining techniques by Feulgen and Rossenbeck in 1924 enabled Feulgen to demonstrate in 1937 that most of the DNA content of the cell is located in the nucleus.
- It was not until the 1950s that the inter-nucleotide bond was established by A.R. Todd (Judson,1979). Because DNA is the only genetic material of most living organisms, so deserves priority in discussion.
Deoxyribonucleic Acid Or DNA
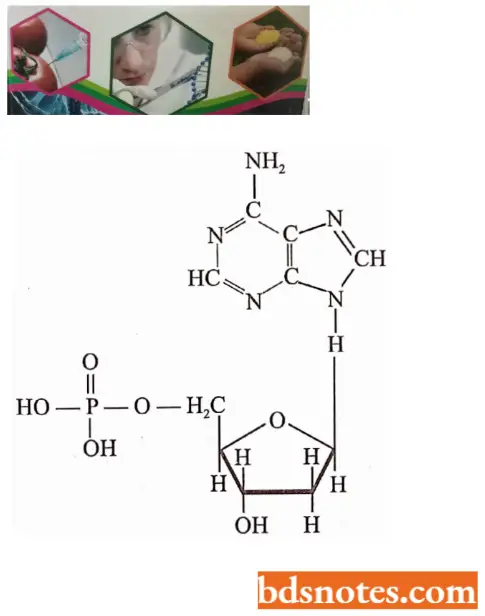
Highly purified DNA, extracted from a wide variety of plants, animals, bacteria, and viruses, is a complex macro-molecular or polymeric chemical compound that contains four kinds of smaller building blocks (monomers) called deoxyribotids or deoxyribonucleotides.
- Each deoxyribonucleotide is made up of three moieties: a phosphoric acid molecule (biologically called phosphate); a pentose sugar called 2 deoxyribose; and pyrimidine and purine nitrogenous bases.
- Four major kinds of nitrogenous bases have been found in four kinds of deoxyribonucleotides of DNA: two are heterocyclic and two-ringed purines, adenine and guanine (G), and two are one-ringed pyrimidines, cytosine and thymine (T). The pyrimidine ring can be numbered in two different ways. In this book, we will follow the old numbering system.
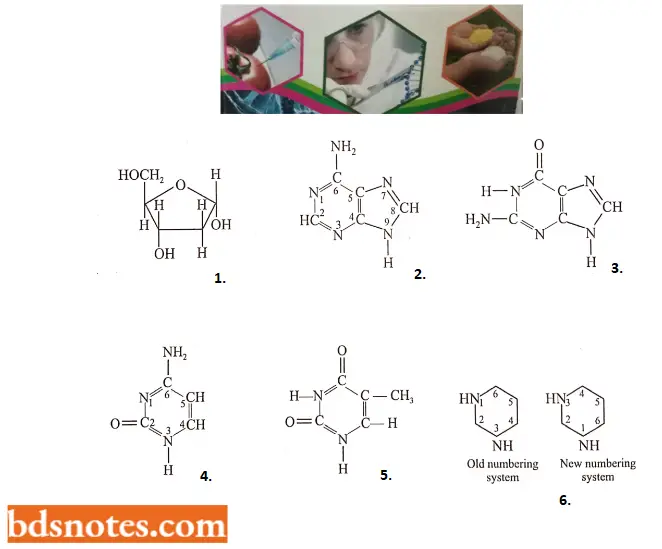
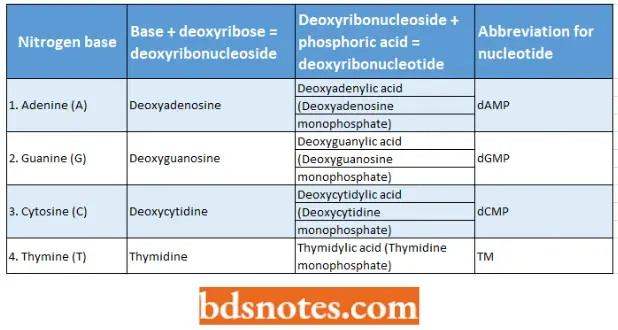
That part of each nucleotide that contains a nitrogenous base and deoxyribose is called deoxyribonucleoside. The four kinds of deoxyribonucleosides and deoxyribonucleotides can be tabulated as follows:
Four nitrogen bases, nucleosides, and nucleotides of the DNA molecule:
- The four deoxyribonucleotides besides occurring in DNA molecules occur in nucleoplasm and cytoplasm but in their triphosphate forms such as deoxyadenosine triphosphate (dATP), deoxyguanosine triphosphate (dGTP), deoxycytidine triphosphate (dCTP) and thymidine triphosphate (TTP).
- The significance of the occurrence of deoxyribonucleotides in triphosphate forms lies in the fact that during DNA replication, the DNA polymerase enzyme can act only on the triphosphate of deoxyribonucleotides.
Molar Ratios of Nitrogen Bases in DNA Molecule
When many samples of DNA were isolated, purified, and analyzed by various techniques, such as paper chromatography, etc., it was found by Hotchkiss and Chargaff in 1948 that contrary to Levene’s (1920’s) tetranucleotide theory which considered DNA as a monotonous polymer having four DNA bases in approximately equal molar proportions, the four nucleotide bases are not necessarily present in DNA in exactly equal proportions.
- Thus, Chargaff reported that the DNA extracted from calf thymus nuclei contains the four bases in the following molar proportions: 28% adenine, 24% guanine, 20% cytosine, and 28% thymine.
- When he analyzed the DNA samples of varied animals, he found that the exact base composition of DNA differs according to its biological source.
- The relative amounts of purines and pyrimidines in samples of DNA of different living organisms are tabulated as follows :
Relative amounts of nitrogen bases in different samples of DNA (Chargaff and Davidson,1955):
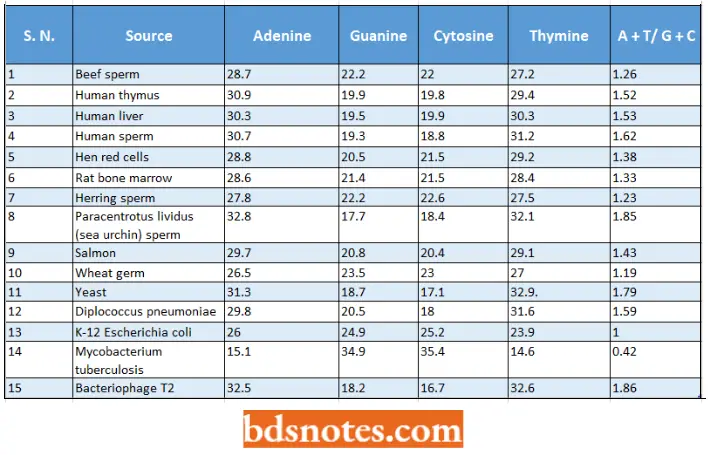
- These different values of A, G, C, and T in different samples of DNA suggest that DNA rather than being a monotonous polymer, carries genetic information in the form of specific nucleotide base sequences.
- The equivalence rule. Chargaff, in 1950, formulated the equivalence rule which suggested that despite wide compositional variations exhibited by different types of DNA, the total amount of purines equaled the total amount of pyrimidines (A + G = T + C); the amount of adenine equaled the amount of thymine (A = T) and the amount of guanine equaled the amount of cytosine (G = C).
- Chargaff’s equivalence rule has been found to apply almost universally in different organisms (viruses, bacteria, plants, and animals).
- However, DNA isolated from higher plants and animals was in general rich in adenine and thymine (A: T) and relatively poor in guanine and cytosine (G: C) (for example, AT/GC ratio of DNA of man was 1.40: 1); whereas DNA isolated from microorganisms (viruses, bacteria and lower plants and animals) was in general rich in guanine and cytosine and relatively poor in adenine and thymine (for example, AT/GC ratio of DNA of Mycobacterium tuberculosis was 0.60: 1).
- These differences in AT/GC ratios of microorganisms and higher organisms undoubtedly reflect the difference in genetic information carried by these hereditary molecules and also their phylogenetic, evolutionary, and taxonomical significance.
Physical, Molecular, Or Geometrical Organization Of DNA: The first person to give any thought to the three-dimensional structure of DNA was W.T.
- Astbury who by his X-ray crystallographic studies of DNA molecules concluded in 1938 that because DNA has high density, so, its polynucleotide was a stack of flat nucleotides, each of which was oriented perpendicularly to the long axis of the molecule and was situated on every 3.4 A° along the stack.
- The X-ray crystallographic studies of Astbury were continued by Wilkins and his associates who managed to prepare highly oriented DNA fibres that allowed them to obtain an X-ray diffraction photograph. One of his female associates Rosalind Franklin obtained a superior X-ray diffraction photograph of DNA which confirmed Astbury’s earlier inference of 3.4 internucleotide distance.
- Such studies demonstrated that DNA was a helical structure with a diameter of 20 A° and a pitch (one round) of about 34 A°.
- Watson and Crick who were already engaged in constructing some suitable model for DNA structure, when observed Franklin’s picture of DNA molecule, they immediately utilized that information in constructing a molecular model for DNA.
- In April 1953, Watson and Crick published their conclusions about the structure of the DNA in the same issue of ‘ Nature’, in which Wilkins and his colleagues presented the X-ray evidence for that structure.
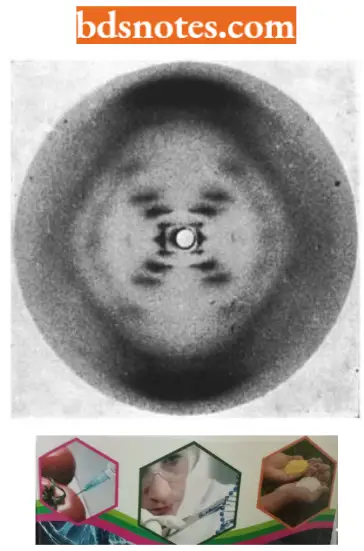
- Considerations of Watson and Crick in the construction of the double-helical structure of DNA molecule. Watson and Crick concluded directly from the X-ray diffraction photograph of DNA taken by Franklin that the DNA polynucleotide chain has the form of a regular helix, the helix has a diameter of about 20A° and, the helix makes one complete turn every 34A° along its length, and hence, since the internucleotide distance is 3.4A°, consists a stack of ten nucleotides per turn.
- Considering the known density of the DNA molecule, Watson and Crick next concluded that the helix must contain two polynucleotide chains, or two stacks of ten nucleotides each per turn since the density of a cylinder 20A° in diameter and 34A° long would be too low if it contained but a single stack of ten, and too high if it contained three or more stacks of ten nucleotides each.
- Before trying to arrange these two polynucleotide chains into a regular helix of the required dimensions, however, Watson and Crick placed a further restriction on their model- a restriction that derived from their knowledge that DNA is, after all, the genetic material.
- If DNA is to contain hereditary information as they reasoned, and if that information is inscribed as a specific sequence of the four bases along the polynucleotide chain, then the molecular structure of DNA must be able to accommodate any arbitrary sequence of bases along its polynucleotide chains.
- Otherwise, the capacity of DNA as an information carrier would be too severely limited. Hence, they felt the construction needed such a regular helix that though is composed of two polynucleotide chains containing an arbitrary sequence of nucleotide bases every 3.4A° along their length, it would, nevertheless, have a constant diameter of 20A°.
- Since the dimension of the purine ring is greater than that of the pyrimidine ring, Watson and Crick hit upon the idea that the two-chain helix could have a constant diameter if there existed a complementary relation between the two nucleotide stacks, so that at every level one stack harbors a purine base and the other a pyrimidine base.
- Finally, to endow the helix with thermodynamic stability, the structure would have ample opportunities for the formation of hydrogen bonds between amino or hydroxyl-hydrogens and keto-oxygens or amino-nitrogens of the purines and pyrimidine bases. These considerations led them to construct a double helix model for the molecular structure of DNA molecules.
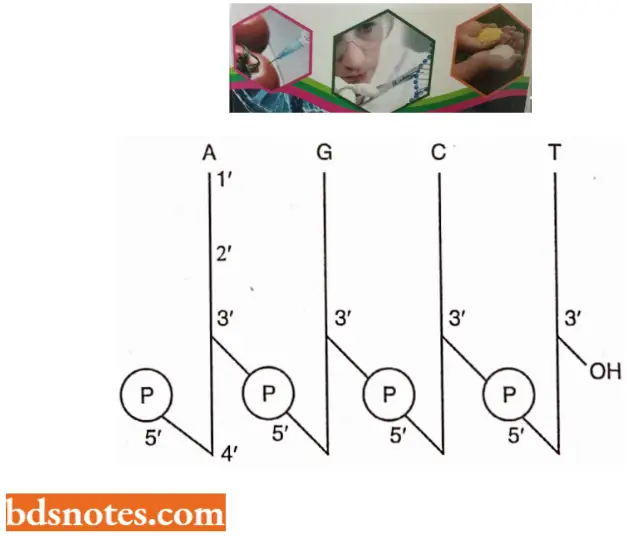
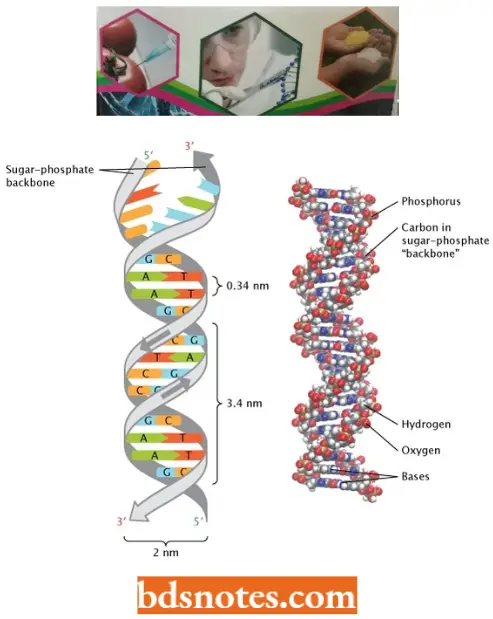
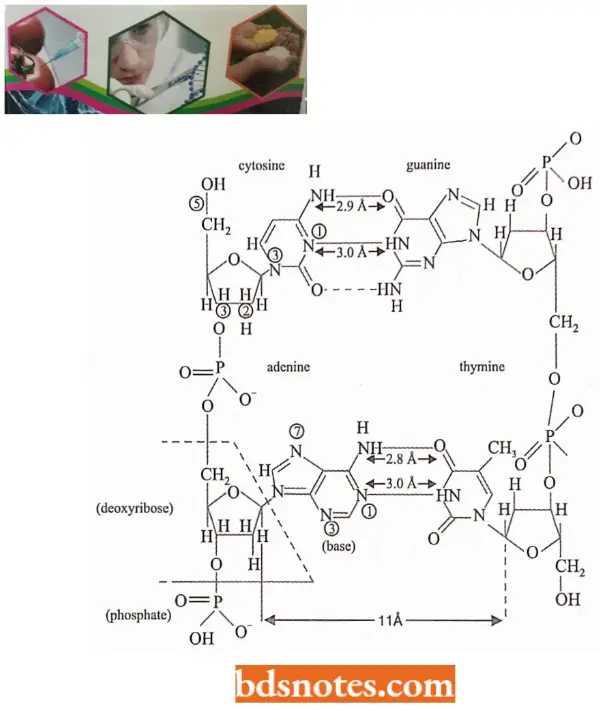
- Watson and Crick’s Structural Model of DNA In a DNA molecule the adjacent deoxyribonucleotides are joined in a chain by phosphodiester bridges or bonds which link the 5′ carbon of the deoxyribose of one mononucleotide unit with the 3′ carbon of the deoxyribose of the next mononucleotide unit.
- According to Watson and Crick DNA molecule consists of two such polynucleotide chains wrapped helically around each other, with the sugar-phosphate chain on the outside (forming the ribbon-like backbone of the double helix) and purines and pyrimidines on the inside of the helix (projecting between two sugar-phosphate backbones as transverse bars).
- The two polynucleotide strands are held together by hydrogen bonds between specific pairs of purines and pyrimidines.
- The hydrogen bonds between purines and pyrimidines are such that adenine can bond only to thymine by two hydrogen bonds, and guanine can bond only to cytosine by three hydrogen bonds and no other alternative is possible between them.
- The specificity of the kind of hydrogen bonds that can be formed assures that for every adenine in one chain, there will be thymine in the other. For every guanine in the first chain, there will be a cytosine in the other, and so on.
- Thus, the two chains are complementary to each other; that is, the sequence of nucleotides in one chain dictates the sequence of nucleotides in the other. The two strands run anti-parallel-that is, have opposite directions.
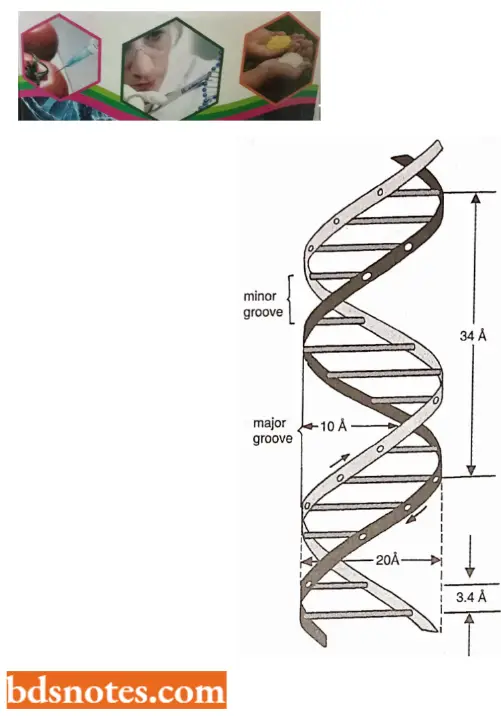
- One strand has phosphodiester linkage in the 3′ → 5′ direction, while the other strand has phosphodiester linkage in just reverse or 5′ → 3′ direction. Further, both polynucleotide strands remain separated by a 20A° distance.
- The coiling of the double helix is right-handed and a complete turn occurs every 34 A°. Since each nucleotide occupies a 3.4 A° distance along the length of a polynucleotide strand, ten mononucleotides occur per complete turn. (The base pairs are rotated 36° concerning each adjacent pair). The helix has two external grooves, a deep wide one, called a major groove and a shallow narrow one, called a minor groove: both of these grooves are large enough to allow protein molecules to come in contact with the bases.
Polymorphism of DNA Helix (Or Alternative Forms of DNA Double Helix)
For about 20 years after the discovery of the DNA double helix in 1953, DNA was thought to have the same monotonous structure, with exactly 36° of helical twist between its adjacent base pairs (10 nucleotide pairs per helical turn) and a uniform helix geometry.
Subsequent experiments have shown that DNA is much more polymorphic than expected.
Thus, DNA can be of the following types:
- The above-described DNA molecule contains the right-handed helical coiling and has been called B-form or B-DNA. It is a biologically important form of DNA that is commonly and naturally found in most living systems.
- This double helical structure of DNA is found to exist in other alternative forms (such as A-form and C-form) which differ in features such as several residues (monomers) per turn (“n”) or the spacing of residues along the helical axis (“h”).
- For example, A-form DNA (A-DNA) is right-handed but less hydrated than the B-form DNA. A- DNA is more compact with 11 base pairs per turn of the helix and it is 23 angstroms in diameter.
- The bases are tilted more about the axis of the helix than in the B-DNA. The A-DNA may occur under experimental conditions.
Important features of different forms of DNA double helical structures:
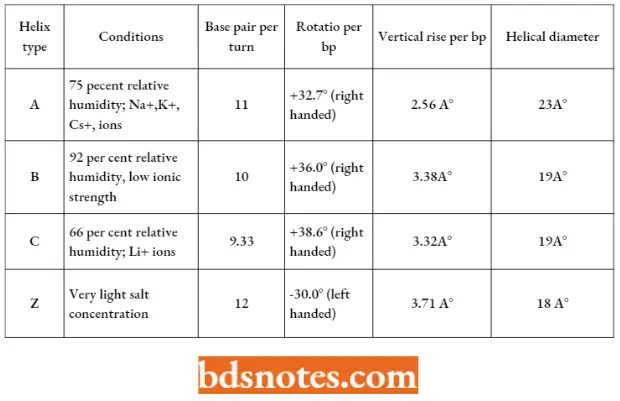
- The B-DNA is found in fibers of living cells at a very high 92 percent relative humidity and low ionic strength.
- Likewise, the A-form of DNA is found at 75 percent humidity in the presence of high ionic strength of NA+, Ka+, or Cs+ ions. C-form of DNA is found at 66 percent relative humidity in the presence of lithium (Li+) ions.
- These three forms are assumed to be found in all DNAs.
- There are certain other forms of DNA such as D-form and E-form, both of which are found as rare extreme variants and contain only 8 and 7 1/2 base pairs per turn respectively.
- These rare DNA variants are found only in some DNA molecules which lack guanine.
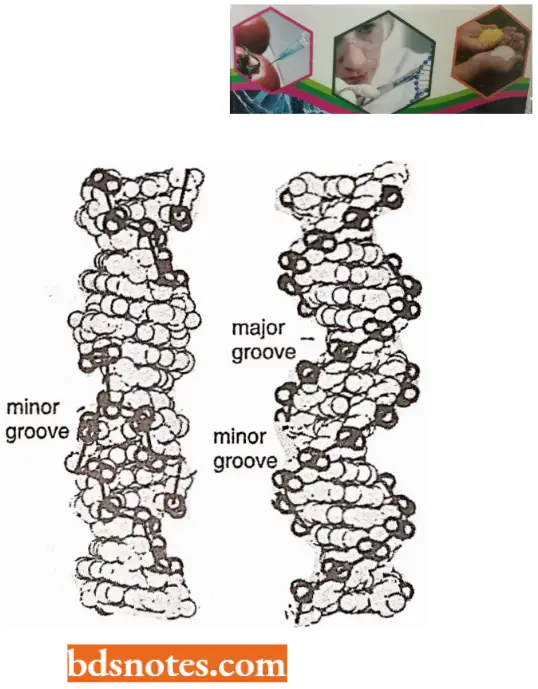
Z-DNA (or Left-handed DNA): Crystallographic studies on synthetic nucleotides consisting of alternating purines and pyrimidines such as GCGCGCGC have shown that lefthanded DNA can also exist.
- This DNA is called Z-DNA because of its zigzag structure. Z-DNA can also be found in solutions of high-ionic strength, such as 2M NaCI.
- In the Z-form of DNA, the molecule still consists of two anti-parallel chains, but, otherwise, it is quite different from the A or B form.
- The helix of Z-DNA is 18 angstrom in diameter, containing 12 base pairs per turn. The differences and similarities of Z-DNA and B-DNA have been summarized.
Similarities between Z-DNA and B-DNA
- Both are double helical.
- In both DNAs, two polynucleotide strands of double helix are antiparallel.
- Both forms exhibit G = C pairing.
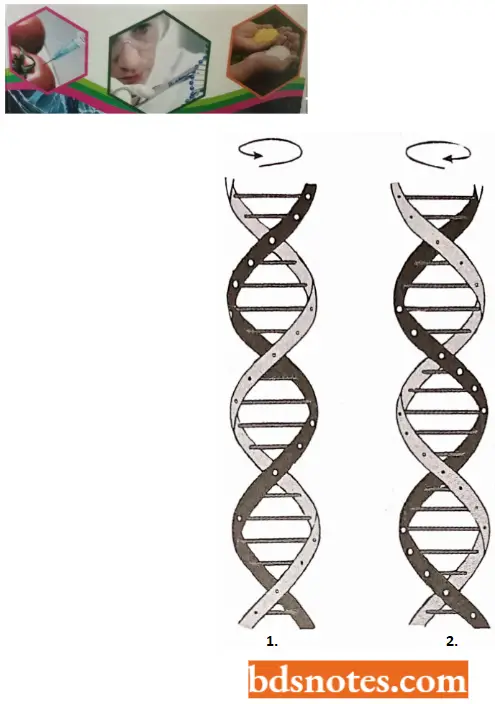
Differences between Z-DNA and B-DNA
- Z-DNA has a left-handed helical sense, while B-DNA has a right-handed helical sense.
- The phosphate backbone of Z-DNA follows a zigzag course, while in B-DNA this backbone is regular.
- In Z-DNA, the adjacent sugar residues have opposite orientations, while in B-DNA they have the same orientation. Due to this, the repeating unit is a dinucleotide in Z-DNA as against a mononucleotide unit in B-DNA.
- In Z-DNA, one complete helix (i.e., a twist through 360°) has twelve base pairs or six repeating dinucleotide units, while in B- DNA one complete helix has only ten base pairs or 10 repeating units.
- The angle of twist (rotation) per repeating unit (dinucleotide) in Z-DNA is 60° than the 36° of mononucleotide in B-DNA.
- In Z-DNA. one complete turn of helix is 45A° long, while in B-DNA it is 34A° long.
- Since bases get more length spread out in Z-DNA and since the angle of tilt is 60°, they are closer to the axis. Due to this fact, the diameter of the Z-DNA molecule is 18 A0 titan the 20 A° diameter of B-DNA.
Regions of Z-DNA may be involved in gene regulation in the cells of “higher” organisms. Short regions of such drastically altered helical geometry could be specifically recognized by gene regulators)’ proteins, and thereby have important biological roles (see Alberts et al., 1989). Lastly, the presence of any alternate configuration (for example, Z-DNA) suggests that DNA is a more flexible molecule than was previously thought and that it can be adopted in the genome of a variety of forms (Rich. 1980).
Ribonucleic Acid (RNA)
Some plant viruses (for example., TMV, turnip yellow mosaic viruses, wound tumor viruses, etc.,), animal viruses (for example, influenza viruses, foot, and mouth viruses; rous sarcoma viruses, poliomyelitis ‘nurses. reoviruses. etc.) and bacteriophages (for example, MS., etc.) contain ribonucleic acid (RNA) as their genetic material.
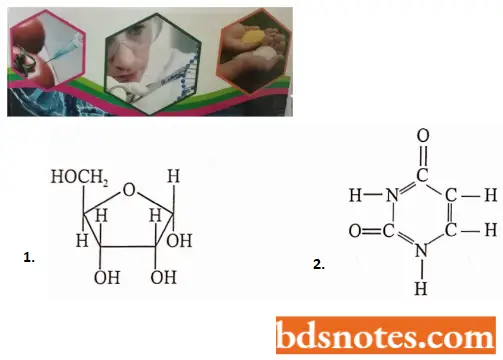
- Like DNA, RNA is polymeric nucleic acid of four monomeric ribotids or ribonucleotides.
- Each ribonucleotide contains a pentose sugar (D-ribose); a molecule of a phosphate group and a nitrogen base.
- The nitrogen bases of RNA are two purines, adenine and guanine, and two pyrimidines. cytosine and uracil.
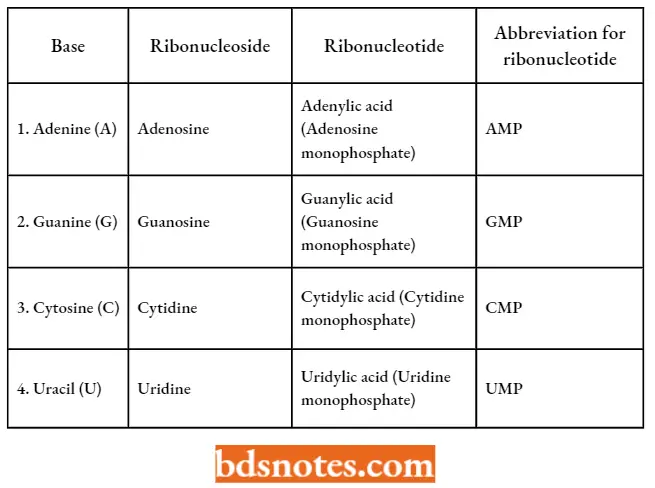
- The four bases, ribonucleotides, and ribonucleotides of RNA can be tabulated as follows:
Four Components Of RNA:
The four ribonucleotides also occur freely in nucleoplasm but in the form of triphosphates of ribonucleosides such as adenosine triphosphate (ATP) and uridine triphosphate (UTP).
Molecular Structure of RNA: RNA molecules may be either single-stranded or double-stranded but not helical like DNA molecules.
Single-stranded RNA occurs as genetic material in plant viruses (for example, TMV, TYM), animal viruses (for example, influenza viruses, foot and mouth viruses, rous sarcoma viruses, poliomyelitis viruses), and bacteriophages (for example., MS2).
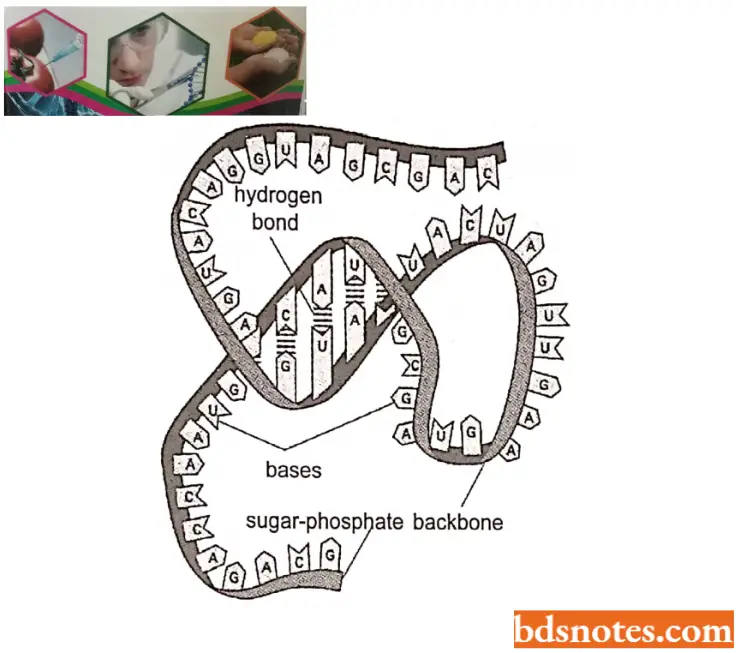
- The non-genetic RNAs except tRNA of prokaryotes and eukaryotes, also have single-stranded RNA molecules. The double-stranded but non-helical RNA occurs as the genetic material in some plant viruses (for example, reoviruses).
- The transfer or soluble RNA (tRNA or sRNA) which is non-genetic RNA of prokaryotes and eukaryotes, is double-stranded but non-helical structure.
- Each strand of RNA is polynucleotidic, that is, made up of many ribonucleotides. In the polynucleotide strand of RNA, the ribose and phosphoric acids of nucleotides remain linked by phosphodiester bonds. The organisms that have only RNA, are called genetic RNA.
- While organisms that have DNA along with RNA, use the RNA in carrying the orders of DNA and in them because RNA has no genetic role, so-called non-genetic RNA. The non-genetic RNA is heterogeneous and includes the following three genera: ribosomal RNA (rRNA), transfer RNA (tRNA), and messenger RNA (mRNA).
- Each genus of non-genetic RNA has DNA-dependent replication (transcription) of itself, that is, it is not self-replicating like DNA and is transcribed by DNA.
Replication of Genetic RNA: The genetic RNA of viruses is self-replicating, that is, it can produce its replica by itself. So, its mode of replication is called RNA-dependent RNA synthesis.
- The genetic research on genetic RNA has revealed the following facts about it.
- The viral RNA functions directly as a messenger RNA which, in association with the ribosomal apparatus of the host, directs the synthesis of both the RNA polymerase enzyme (required for RNA replication) and the proteins of the viral coat.
- With the mediation of RNA polymerase and on the standard base-pairing principles, the viral RNA serves as a template in the synthesis of a complementary RNA chain, and thus a double-stranded structure is produced.
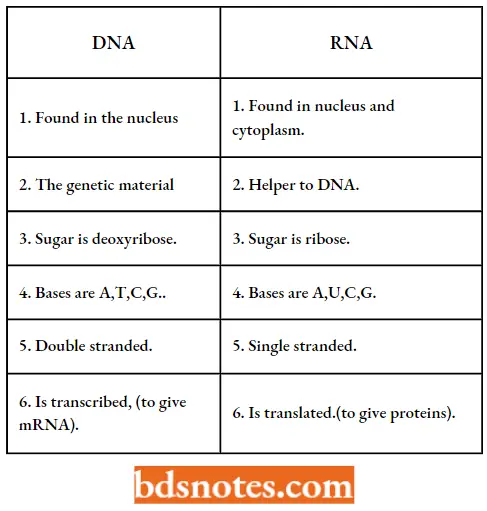
RNA similarities and differences. (Source: Sylvia S.Mader, 1998):
A.DNA – RNA similarities
- Both are nucleic acids.
- Both are composed of nucleotides.
- Both have a sugar-phosphate backbone.
Both have four different types of bases.
DNA – RNA differences:
Chemical Nature Of The Genetic Materials Questions And Answers
Question 1. Assume the following base sequence was found in a 20-base DNA strand : 3′ ATT CGA CCT TAT TAC TGC AC 5′
- What would be the first 5 bases in the 3′ end of the complementary strand?
- What would be the 10 bases of the 5′ end of the complementary strand?
- Assuming the presence of complementary strands, what is the percent composition of the polymer concerning AT base pairs and GC base pairs?
Answer:
- Bases at the 3′ end of the opposite strand will be complementary to those at the end 5’
end of the polymer given. Therefore, the first 5 bases will be 3′ GTGCA. - 5′ TAA GCT GGAA
- A-T = 60 per cent, G-C = 40 per cent.
Question 2. Analysis of four double-stranded DNA samples yielded the following information: 1.15% cytosine; 2.12% guanine; 3.35% thymine; and 4.28% adenine.
- What would be the percentage of the other bases in each sample?
- Could any of these samples have been obtained from the same organism? If so, which ones?
Answer:
- 15% C, 15% G, 35% T, 35% A.
- 12% G, 12% C, 38% T, 38% A.
- 35% T, 35% A, 15% C, 15% G.
- 28% A, 28% T, 22% G, 15% C.
Yes, samples 1 and 3.
Question 3. The ratio of the bases present in different samples of nucleic acid yielded the following results
- (A + C)/(T + G) = 1,
- (A + C)/(U + G) = 0.8,
- (A + G)/(T + C) = 1.5.
Which were RNA and which were DNA? Which were single and double-stranded?
Answer:
- Double-stranded DNA,
- Single-stranded RNA,
- Single-stranded DNA.
Question 4. If one DNA sample had a melting temperature of 85.5°C and another showed a melting temperature of 88°C. What might you conclude concerning the base composition of the two samples?
Answer: The second sample has a higher G-C content.
Question 5. Assume an average-sized gene consisting of a linear sequence of 1000 bases and there were 1000 genes in a bacterial chromosome.
- How many bases would such a chromosome contain in each strand of the double helix?
- If 10 nucleotides = 34A°, how long would this chromosome would be in millimeters?
Answer:
- 1 million,
- 3.4 mm.
Question 6.
- What background material did Watson and Crick have available for developing a model of DNA?
- What was their contribution to the building of the model?
Answer:
- The ladderlike pattern was known from X-ray diffraction studies. Chemical analyses had shown that a 1:1 relationship existed between the organic bases adenine and thymine and between cytosine and guanine. Physical data concerning the length of each spiral and the staking of bases were also available.
- Watson and Crick developed the model of a double helix, with the rigid strands of sugar and phosphorus forming spirals around an axis, and hydrogen bonds connecting the complementary bases in base pairs.
Question 7.
- Why was a double helix chosen for the basic pattern of the molecule?
- Why were hydrogen bonds placed in the model to connect the base?
Answer:
- A multistranded, spiral structure was suggested by the X-ray diffraction patterns. A double-stranded helix with specific base-pairing fits the 1:1 stoichiometry observed for A: T and G: C in DNA (stoichiometry is a branch of chemistry that treats the proportions of elements or compounds involved in reactions, and the methods of calculating them).
- The use of the known hydrogen-bonding potential of the bases provided a means of holding the two complementary strands in a stable configuration in such a double helix.
Question 8. What are the differences between DNA and RNA?
Answer: DNA has one atom less of oxygen than RNA in the sugar part of the molecule. In DNA, thymine replaces the uracil that is present in RNA. (In certain bacteriophages, DNA containing a uracil is present). DNA is most frequently double-stranded, but bacteriophages such as Φ x 174 contain single-stranded DNA. RNA is most frequently single-stranded. Some viruses, such as reoviruses, however, contain double-stranded RNA chromosomes.
Question 9. RNA was extracted from TMV (tobacco mosaic virus) particles and found to contain 20 percent cytosine. Using this information, is it possible to predict what percentage of the bases in TMV are adenine? If so, what percentage? If not, why not?
Answer: No. TMV RNA is single stranded. Thus, the base-pair stoichiometry of DNA does not apply.
Chemical Nature Of The Genetic Materials Multiple Choice Questions Answers
Question 1. In a sample, DNA is found to have the base composition (mole ratio) of adenine = 40, T = 22, G = 21, and cytosine = 17. It shows that
- DNA is a circular duplex
- DNA is a linear duplex
- DNA is single-stranded
- DNA has a melting point
Answer: 3. DNA is single-stranded
Question 2. In the double helix model of DNA, how far is each base pair from the next base pair?
- 3.4 nm
- 0.34 nm
- 2.0 nm
- 34 nm
Answer: 2. 0.34 nm
Question 3. The scientist who developed the cytochemical technique for the identification DNA was
- McClintock
- Abbe
- Fuelgen and Rossenbeck
- Waldeyer
Answer: 3. Fuelgen and Rossenbeck
Question 4. What is a common point of similarity between DNA and RNA?
- Both are double-stranded
- Both have identical sugar molecules
- Both have identical pyrimidine bases
- Both are polymers of nucleotides
Answer: 4. Both are polymers of nucleotides
Question 5. DNA strands are antiparallel because of
- Hydrogen bonds
- Phosphodiester bonds
- Disulfide bonds
- Glycosidic bonds
Answer: 2. Phosphodiester bonds
Question 6. Chargaff’s rule applies to
- Single-stranded RNA
- Single-stranded DNA and RNA
- Single-stranded DNA
- Double-stranded DNA
Answer: 4. Double-stranded DNA
Question 7. The diameter of the Z-DNA molecule is
- 18A°
- 22A°
- 45A°
- 34A°
Answer: 1. 18A°
Question 8. What is the type of coiling in DNA?
- Right-handed
- Left-handed
- Opposite
- Zig-zag
Answer: 1. Right-handed
Question 9. Diameter of DNA helixes
- 20A°
- 10A°
- 30A°
- 40A°
Answer: 1. 20A°
Question 10. Which ratio is constant for DNA?
- A + G/T+C
- A + T/G + C
- A + C/U + G
- A + U/C + G
Answer: 1. A + G/T+C

Leave a Reply