Chelating Agents
Question 1. Write Drug Treatment Of BAL.
Or
Write A Short Note On BAL.
Or
Write A Short Note On British AntiLewisite.
Answer:
The term BAL refers to British until-Lewisite or Dimercaprol.
- BAL is an oily pungent, smelling, viscous liquid developed during World War I by British as an antidote to arsenical war gas lewisite.
- Two SH groups of dimercaprol bind those metals which produce their toxicity by interacting with sulfhydryl-containing enzymes in the body, i.e. arsenic, mercury, gold, bismuth, nickel, and copper.
Read And Learn More: Pharmacology Question And Answers
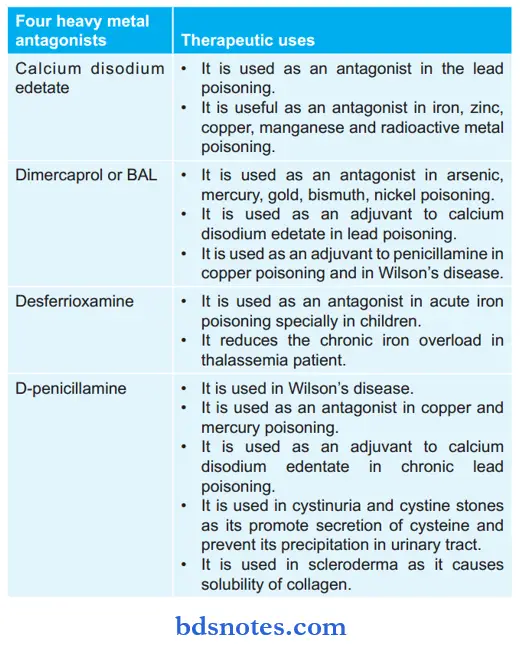
British AntiLewisite Uses
- It is used in poisoning by arsenic, mercury, gold, bismuth, nickel, and copper.
- It is used as an adjuvant to calcium disodium edetate in lead poisoning.
- It is used as an adjuvant to penicillamine in copper poisoning and Wilson’s disease.
British AntiLewisite Contraindications
It is contraindicated in iron and cadmium poisoning because the dimercaprol-Fe and dimercaprol-Cd complex is itself toxic.
British AntiLewisite Adverse Effects
Rise in BP, tachycardia, vomiting, tingling, burning sensations, inflammation of the mucous membrane, sweating, cramps, headache, and anxiety.
Question 2. Write A Short Note On Edta.
Answer:
The full form of EDTA is ethylenediaminetetraacetic acid.
- It is given as a calcium disodium salt to prevent life-threatening depletion of calcium.
- It is poorly absorbed orally and is given either by slow intravenous infusion or by IM route
- It is used chiefly for the chelation of lead, but it can also be used in poisoning by zinc, manganese, and certain heavy radionucleotides: uranium, plutonium, etc.
- Nephrotoxicity from EDTA has been reported, but it can be maintained by adequate urine flow, avoidance of excessive doses, and limitation treatment course to 5 or fewer consecutive days.
- The other side effects are fever, fatigue, myalgia, headache, nausea, vomiting, etc.
Question 3. Write A Short Note On Desferrioxamine.
Answer:
Desferrioxamine which has a very high affinity for iron is capable of chelating 85 mg of elemental iron.
- The straight-chain desferrioxamine molecule winds around ferric iron and forms a stable nontoxic complex that is excreted in the urine. It removes loosely bound
iron as well as that from hemosiderin and ferritin, but not from hemoglobin or cytochrome. - Another desirable property is its low affinity for calcium.
Edta Uses
- Acute iron poisoning: IV desferrioxamine is given.
- Transfusion siderosis: This occurs in thalassemia patients who receive repeated blood transfusions. Desferrioxamine 0.5–1 g/day IM helps to excrete the chronic iron overload; may also be infused IV concurrently with a blood transfusion of 2 g per unit of blood.
- It is used IV to chelate aluminum during dialysis.
Edta Adverse Effects
- Desferrioxamine can cause histamine release and fall in BP, flushing, itching urticaria, and rashes.
- A variety of allergic reactions are reported.
- Changes in the lens and retina can occur on repeated use.
- Other side effects are abdominal pain, loose motions, muscle cramps, fever and dysuria, diarrhea, dyspnea, hypotension, and tachycardia.
- Long-term use can cause neurotoxicity.
Question 4. Enumerate Four Chelating Agents.
Answer:
The four chelating agents are as follows:
- Dimercaprol or BAL
- Disodium edentate
- Calcium disodium edentate
- Deferiprone
Question 5. Enumerate Four Heavy Metal Antagonists And Their Therapeutic Uses. Describe The Mechanism Of Action Of Heavy Metal Antagonists.
Answer:
Enumeration of Four Heavy Metal Antagonists Along with Their Therapeutic Uses
Mechanism Of Action Of Heavy Metal Antagonists
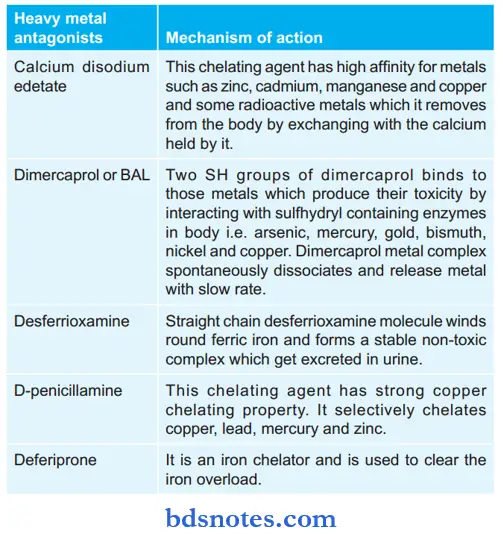
Heavy metals exert their toxic effects by combining with and inactivating the functional groups (-SH, -S-S-, -NH2, -OH, -PO3) of vital enzymes.
In this way, they interfere with the normal physiological functions of these enzymes in the body leading to toxic effects. These groups are called “ligands.”
Metal poisoning is corrected by using chelating agents. A chelating agent consists of polar groups such as -SH, -OH, -NH2, or -COOH, which can bind a metal ion.
Chelating agents, therefore, compete with body ligands (for heavy metal binding) to form stable, non-toxic, water-soluble complexes, which are then easily excreted from the body.
Following is the mechanism of action of various heavy metal antagonists:

Leave a Reply