Cell Injury And Cellular Adaptations
Question 1. Write Briefl On Dystrophic Calcification.
Or
Write A Short Note On Dystrophic Calcification.
Answer:
Dystrophic calcification is characterized by the deposition of calcium salts in dead or degenerated tissues with normal calcium metabolism and normal serum calcium levels.
Dystrophic Calcification Pathogenesis
The process of dystrophic calcification involves two phases, i.e. initiation and propagation.
- Initiation: It is the phase in which calcium and phosphates begin to accumulate intracellularly in the mitochondria, or extracellularly in membrane-bound vesicles.
- Propagation: It is the phase in which minerals deposited in the initiation phase are propagated to form mineral crystals.
Dystrophic Calcification Histology
- With hematoxylin and eosin stains, the calcium salts have a basophilic, amorphous granular, and sometimes clumped appearance.
- At times heterotrophic bone formation may occur.
- Progressive acquisition of outer layers may create lamellated configurations, called psammoma bodies.
Read And Learn More: Pathology Question And Answers
Dystrophic Calcification Examples
Calcification in Dead Tissue
- Caseous necrosis in tuberculosis is the most common site for dystrophic calcification.
- Liquefaction necrosis may get calcified.
- Fat necrosis in the breast or pancreatitis results in the deposition of calcium salts.
- Calcification in breast cancer.
- Infarcts may sometimes undergo dystrophic calcification.
Calcification in Degenerated Tissue
- Dense old scars, atheromas, and stroma of tumors may undergo calcification.
- Calcinosis cutis is a condition in which there is irregular nodular deposition of calcium salts in the skin and subcutaneous tissue.
- Senile degenerative changes: Dystrophic calcification in costal cartilages, tracheal or bronchial cartilages, and pineal gland in the brain.
Question 2. Write A Short Note On.
1. Metaplasia
2. Apoptosis
Or
Write A Short Note On Metaplasia.
Answer:
1. Metaplasia
Meta means transformation and plasma means growth. Metaplasia is defined as a reversible change of one type of epithelial or mesenchymal adult cells to another type of adult epithelial or mesenchymal cells usually in response to an abnormal stimulus.
Metaplasia is divided into two types
1. Epithelial metaplasia: This is a more common type. The metaplastic change may be patchy or diffuse. Some common types of epithelial metaplasia are:
- Squamous metaplasia: There is a transformation of various types of epithelium into squamous epithelium due to chronic irritation.
- In bronchus (normally lined by stratified columnar ciliated epithelium) in chronic smokers.
- In the gallbladder, in prostrate in chronic prostatitis.
- Columnar metaplasia: There is a transformation of various epithelium into columnar epithelium.
- Intestinal metaplasia in healed chronic gastric ulcer.
- Conservation of pseudostratified columnar epithelium into chronic bronchitis to columnar type.
2. Mesenchymal metaplasia: There is the transformation of one adult type of mesenchymal tissue into another.
- Osseous metaplasia: This is the formation of bone in fibrous tissue, cartilage, and myeloid tissue.
- Arterial wall in old age
- In the fibrous stroma of the tumor.
- In cartilage of larynx and bronchi in old age.
- Cartilaginous metaplasia: In the healing of the fracture.
2. Apoptosis
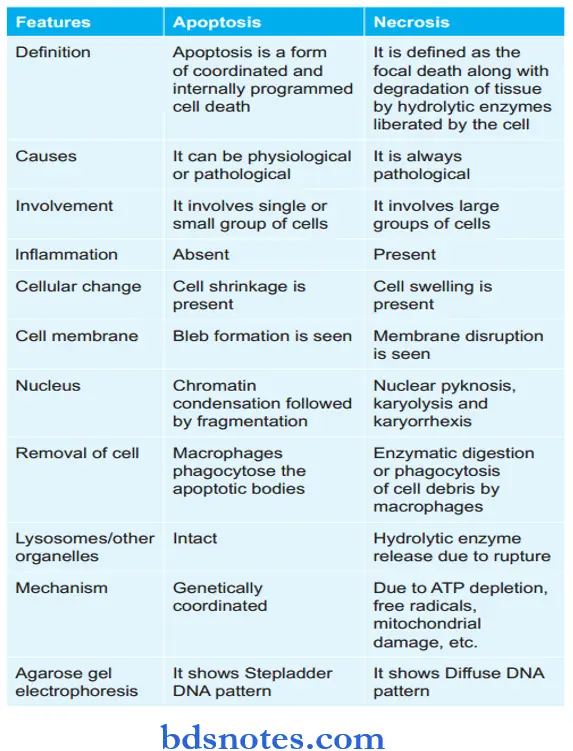
- Apoptosis is a form of “coordinated and internally programmed cell death.”
- Apoptosis occurs in physiologic and pathologic conditions.
- Physiologic conditions:
- Organized cell destruction during the development of the embryo.
- Endometrial shedding, replacement proliferation such as in intestinal epithelium.
- Involution of the thymus at an early age.
- Pathologic conditions: Cell death in tumors, in immune mechanisms, in viral infections, in injury, etc.
Pathologic Changes During Apoptosis
- The apoptotic cells are round to oval.
- Shrinkage of cells with dense cytoplasm.
- Chromatin condensation around the periphery of the nucleus.
- Convolutions of cell membranes with the formation of membrane-bound spherical bodies called apoptotic bodies.
Mechanism Of Apoptosis
Question 3. Write A Short Note On Dysplasia.
Or
Write Note On Dysplasia.
Answer:
Dysplasia means” disordered cellular development”.
- It is also referred to as atypical hyperplasia.
- Dysplasia occurs most often in epithelial cells.
- Causes of dysplasia include diverse cellular insults including physical, chemical, and biological.
- Epithelial dysplasia is characterized by cellular proliferation and cytological changes.
- These changes include:
- Increased number of layers of epithelial cells.
- Disorderly arrangement of cells.
- Loss of basal polarity, i.e. nuclei lying away from the basement membrane.
- Cellular and nuclear pleomorphism, i.e. altered cellular and nuclear size and shape.
- Increased nuclear: cytoplasmic ratio.
- Nuclear hyperchromatism, i.e. increased basophilia on staining with hematoxylin.
- Increased mitotic activity, i.e. altered cell proliferation.
- Dysplasia occurs due to chronic irritation or prolonged inflammation.
- Dysplasia progresses into carcinoma.
- The two most common examples of dysplastic changes are the Uterine cervix and the respiratory tract.
Question 4. Write The Difference Between Dystrophic And Metastatic Calcification.
Answer:
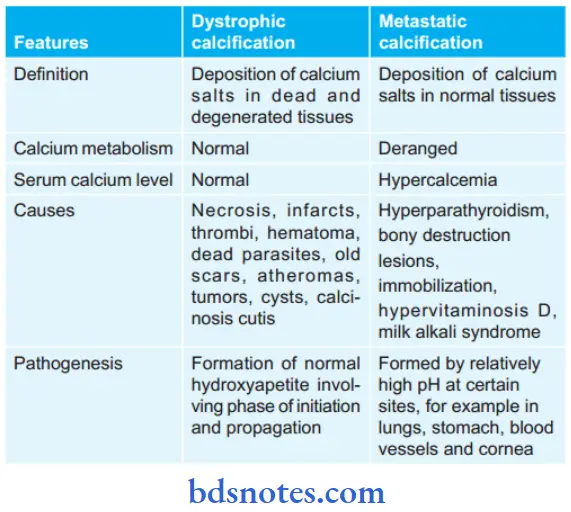
Question 5. Write A Short Note On Fatty Changes.
Or
Write In Short About Fatt Liver.
Or
Write a Short Note On Fatt Degeneration.
Or
Write A Note On Fatt Liver.
Or
Define Degeneration And Give In Detail The Etiology, Pathogenesis, And Gross And Microscopic Changes Of Fatt Liver.
Answer:
Fatt change is the intracellular accumulation of neutral fat within parenchymal cells.
- Fatt change neither involves degeneration nor it involves infiltration.
- The deposition of fat is in the cytosol and it represents an acute increase in intracellular lipids.
- It is common in the liver and can occur in any non-fatty tissues such as the heart, skeletal muscle, kidney, liver, etc.
Fatty Liver
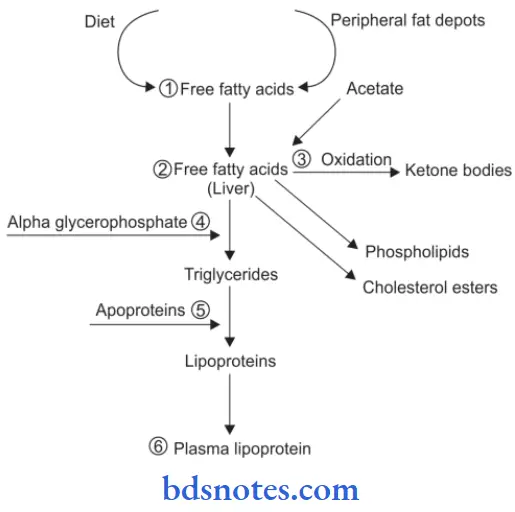
Fatty Liver Pathogenesis
In fatty liver, accumulation of triglycerides can occur due to the following defects in normal fat metabolism:
- Increased entry of free acids into the liver.
- Increased synthesis of fatty acid by the liver.
- Decreased oxidation of fatty acids into ketone bodies.
- Increasedglycerophosphatecausingincreasedesterifiation of fatty acids to triglycerides.
- Decreased synthesis of “lipid acceptor protein” so decreased formation of lipoprotein from triglycerides.
- Block in the excretion of lipoprotein from the liver into plasma.
Fatty Liver Etiology
- Excess alcohol consumption
- Starvation
- Malnutrition
- Obesity
- Diabetes mellitus
- Chronic illness (tuberculosis)
- Late pregnancy
- Hepatotoxins such as CCl4, ether
- Certain drugs such as steroids, tetracyclin, and aspirin
- Hypoxia in anemia and cardiac failure.
Fatty Liver Gross Changes
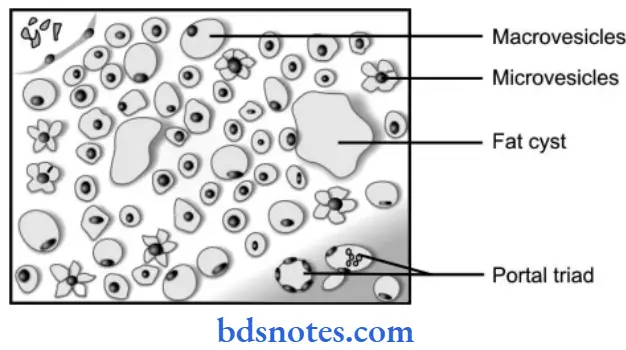
Grossly, the fatty liver is enlarged, pale, soft, and yellowish with a tense glistening capsule and rounded margins. The focal fatty change is yellow mottling.
Gross Changes Microscopically
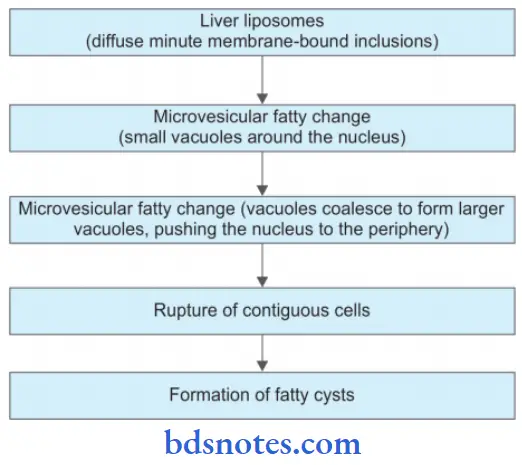
- The lipid vacuoles are initially small and are present around a nucleus.
- With the progression of the process, the vacuoles become large pushing the nucleus to the periphery of the cell.
- At times, the hepatocytes laden with large lipid vacuoles may rupture and lipid vacuoles coalesce to form fatty cysts.
Events In Evaluation Of Fatty Liver
Question 6. Write A Short Note On Gangrene.
Or
Write In Brief On Gangrene.
Or
Defie Gangrene And Describe Wet Gangrene.
Answer:
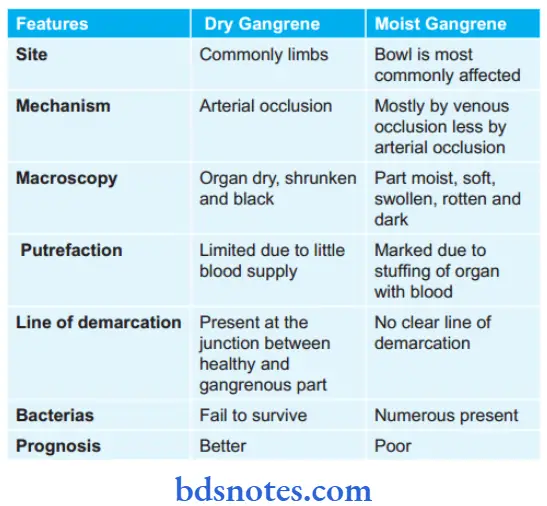
Gangrene is the necrosis of tissue that is associated with super-added putrefaction, most often following coagulative necrosis due to ischemia, For Example. gangrene of the bowl, gangrene of the limb, etc. There are three main forms of gangrene, i.e. dry, wet, and gas gangrene.
1. Dry Gangrene
- This form of gangrene begins in the distal part of a limb due to ischemia. For example in toes and feet due to arteriosclerosis and ergot poisoning.
- Other causes of dry gangrene foot include Buerger’s disease, Raynaud’s disease, and trauma.
- Dry gangrene starts in one of the toes which is farthest from the blood supply and has so small amount of blood that invading bacteria find very hard to grow in the necrotic area.
- Dry gangrene spread slowly upwards till it reaches a point where the blood supply is adequate to keep tissue viable.
- The characteristic appearance of this gangrene is the formation of a line of separation between the gangrenous and viable parts.
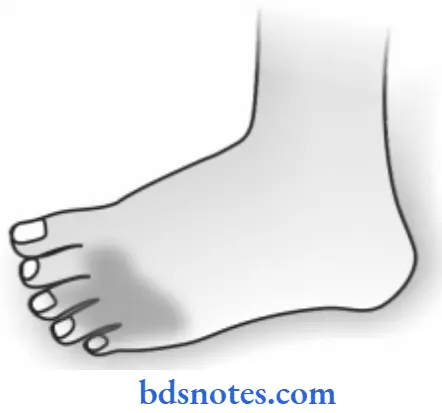
Dry Gangrene Pathologic Changes
- The affected part is dry, shrunken, and dark black.
- The line of separation usually brings about complete separation.
- The line of separation consists of inflammatory granulation tissue.
2. Wet Gangrene
This occurs in naturally moist tissues and organs such as the bowel, mouth, lung, cervix, vulva, etc.
- Two other examples of clinical significance are:
- Diabetic foot due to high glucose content in necrosed tissue which favors the growth of bacteria.
- Bedsore in a bedridden patient.
- Wet gangrene occurs due to blockage of venous and arterial blood flow. The affected part is stuffed with blood which favors rapid growth of putrefactive bacteria. Toxic products formed by bacteria get absorbed causing the systemic manifestation of septicemia and finally death.
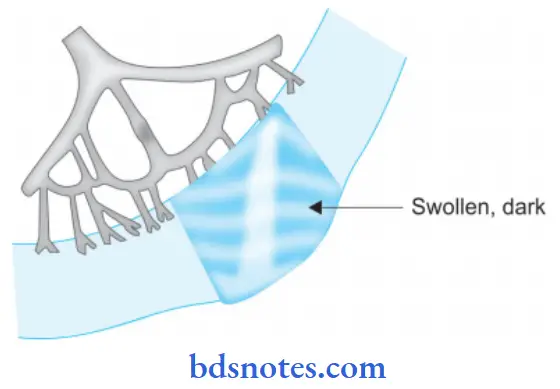
Wet Gangrene Pathologic Changes
- The affected part is soft, swollen, putrid, rotten, and dark.
- No clear line of demarcation.
Wet Gangrene Histologically
- Presence of coagulative necrosis with the stuff of the affected part with blood.
- Mucosa is ulcerated and sloughed.
- There is the presence of intense acute inflammatory exudates and thrombosed vessels.
- The line of demarcation between the gangrenous segment and the viable bowel is not clear-cut.
3. Gas Gangrene
- It is a special form of wet gangrene caused due to gas forming clostridia.
- Clostridia gain entry from the open contaminated wounds into the tissues mainly in muscles.
- Clostridia produce various toxins which produce necrosis and edema locally and are also absorbed producing profound systemic manifestations.
Gas Gangrene Gross Changes
- The affected area is swollen, edematous, painful, and crepitant due to the accumulation of gas bubbles in tissues.
- The affected area becomes dark black and foul smelling.
Gas Gangrene Histologically
- Muscle fibers undergo coagulative necrosis along with liquefaction.
- A large number of Gram-positive bacilli can be identified.
- At the periphery, a zone of leucocyte infiltration, edema, and congestion are found.
- Capillary and venous thrombi are commonly seen.
Question 7. Define Necrosis. Describe All Types Of Necrosis In Detail.
Or
Define Necrosis And Write In Detail The Types Of Necrosis.
Or
Define Necrosis And Its Types In Detail.
Or
Define Necrosis And Types Of Necrosis Giving Examples Of Each Type Briefl.
Or
Write A Short Note On Types Of Necrosis.
Or
Define Necrosis. Enumerate The Type And Discuss Caseous Necrosis.
Or
Define Necrosis. Enumerate Types Of Necrosis And Describe Caseous Necrosis.
Or
Define And Enumerate Types Of Necrosis. Describe Caseous Necrosis.
Answer:
Necrosis is defined as focal death along with the degradation of tissue by hydrolytic enzymes liberated by dead cells. It is invariably accompanied by an inflammatory reaction.
Type Of Necrosis
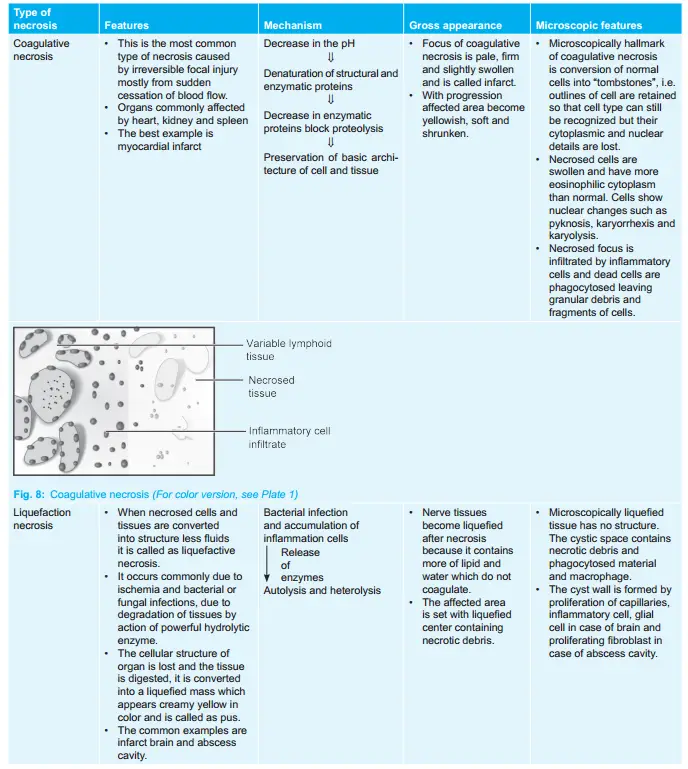
Morphologically necrosis is classified into five distinct types:
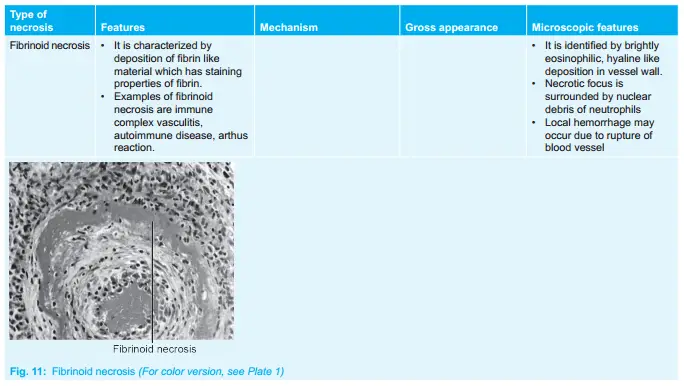
Question 8. Write A Short Note On Coagulative Necrosis.
Answer:
It is characterized by the deposition of fibrin-like material which has the staining properties of firing.
Examples of fibrinoid necrosis are immune complex vasculitis, autoimmune disease, and Arthus reaction.
Coagulative Necrosis Mechanism
Coagulative Necrosis Gross Appearance
- The focus of coagulative necrosis is pale, firm, and slightly swollen and is called an infarct.
- With progression, the affected area becomes yellowish, soft, and shrunken.
Coagulative Necrosis Microscopic Features
- Microscopically hallmark of coagulative necrosis is the conversion of normal cells into “tombstones”, i.e. outlines of cells are retained so that cell type can still be recognized but their cytoplasmic and nuclear details are lost.
- Necrosed cells are swollen and have more eosinophilic cytoplasm than normal. Cells show nuclear changes such as pyknosis, karyorrhexis, and karyolysis.
- Necrosed focus is infiltrated by inflammatory cells and dead cells are phagocytosed leaving granular debris and fragments of cells.
Question 9. Write In Short About Caseous Necrosis.
Or
Write Briefl On Caseation Necrosis.
Answer:
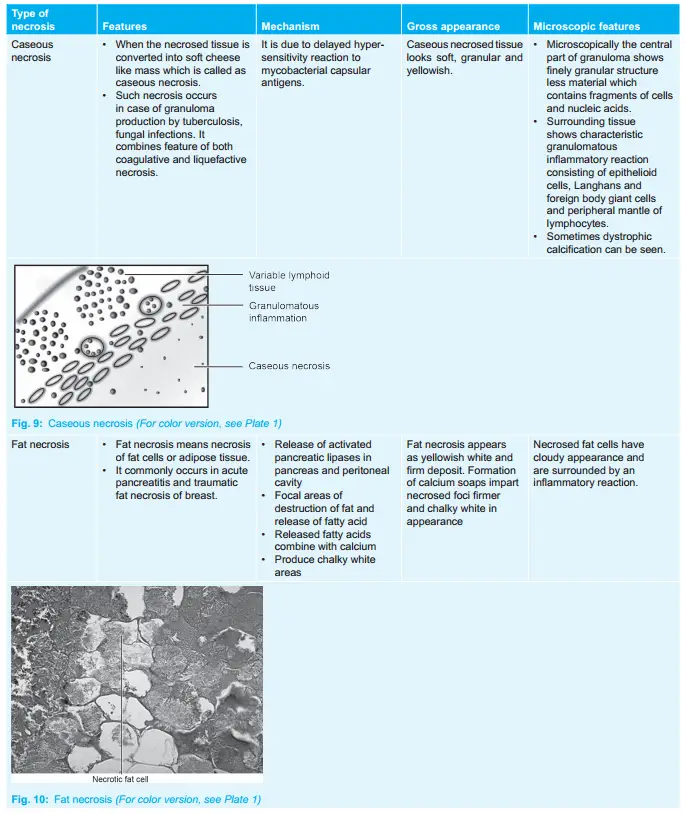
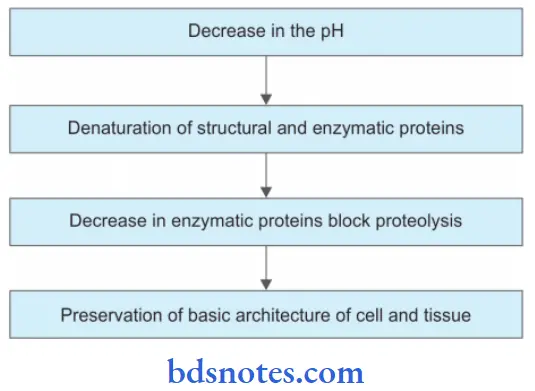
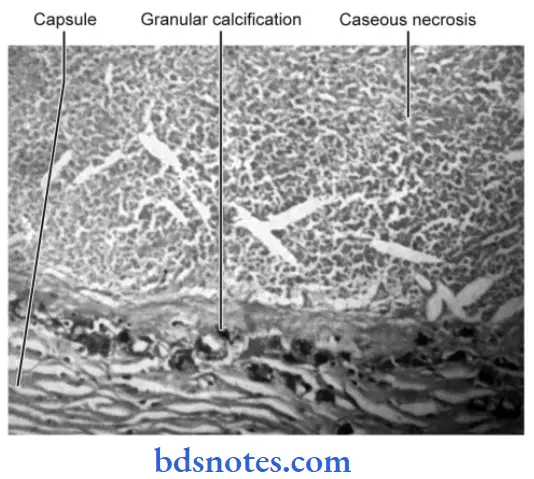
When the necrosed tissue is converted into a soft cheese-like mass it is called as caseous necrosis.
- Such necrosis occurs in case of granuloma production by tuberculosis, and fungal infections.
- It combines features of both coagulative and liquefactive necrosis.
Caseous Necrosis Pathogenesis
It is due to delayed hypersensitivity reaction to mycobacterial capsular antigens.
Caseous Necrosis Gross Appearance
Caseous necrosed tissue looks soft, granular, and yellowish and resembles as dry cheese. Its appearance is due to hytotoxic effects of lipopolysaccharides present in the capsule of M. tuberculum.
Caseous Necrosis Microscopic Appearance
- Microscopically the central part of granuloma shows a finely granular structure with less material that contains fragments of cells and nucleic acids.
- Surrounding tissue shows characteristic granulomatous inflammatory reactions consisting of epithelioid cells, Langhans and foreign body giant cells, and peripheral mantle of lymphocytes.
- Sometimes dystrophic calcification can be seen.
Question 10. Write A Short Note On Necrosis.
Answer:
Necrosis is defined as focal death along with the degradation of tissue by hydrolytic enzymes liberated by dead cells. It is invariably accompanied by an inflammatory reaction.
- Necrosis can be caused by ischemia, infection, poisoning, etc., and is invariably pathological.
- Necrosis usually precipitates an inflammatory response and is accompanied by cell swelling, lysis, and lysosomal leakage. Self-digestion of cells by enzymes liberated from their own lysosomes is known as autolysis.
- Following are the events in necrosis:
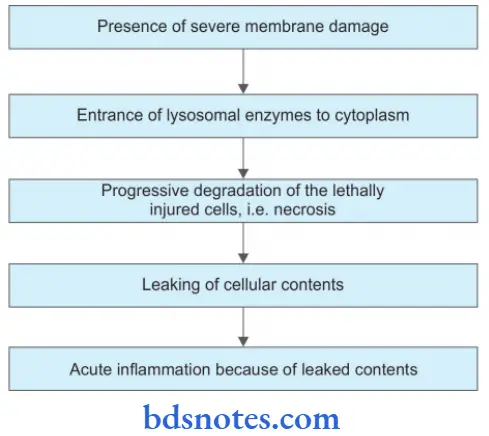
Various Cytoplasmic And Nuclear Changes In Necrosis
Necrosis Cytoplasmic Changes
- Increased eosinophilia of the cytoplasm because:
- Loss of normal cytoplasmic basophilia caused by the loss of RNA
- Denaturation of cytoplasmic proteins which then bind strongly to the eosin dye.
- Presence of glassy homogenous cytoplasm due to loss of glycogen.
- There is swelling as well as vacuolation of the cytoplasm.
- Swelling of cells and organelle may eventually lead to discontinuities in cell and organelle membranes and finally rupture.
- There is the formation of myelin figures, i.e. phospholipid masses derived from damaged cell membranes.
Necrosis Nuclear Changes
The changes in the nucleus appear in one of the following three patterns:
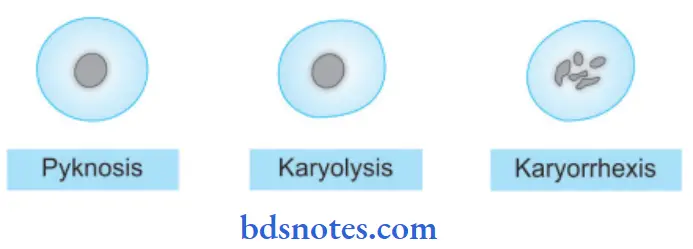
- Shrinkage of the nucleus and increased basophilia, i.e. pyknosis
- Nuclear fragmentation, i.e. karyorrhexis
- The nucleus undergoes dissolution, i.e. karyolysis.
Question 11. Write The Method Of the Spread Of Gas Gangrene And Write The Staining And Morphology Of the Organism Producing It.
Answer:
Method Of Spread Of Gas Gangrene
Clostridia release collagenase and hyaluronidase that degrade extracellular matrix proteins and contribute to bacterial invasiveness, but their most powerful virulence factors are the many toxins they produce. C. perfringens secretes 12 toxins, the most important of which is alpha toxin.
Alpha toxin is a phospholipase C that degrades lecithin, a major component of the cell membrane, and so destroys red blood cells, platelets, and muscle cells, causing myonecrosis.
The alpha toxin also has a sphingomyelinase activity that contributes to nerve sheath damage. The O toxin binds cholesterol and forms a membrane-destabilizing pore that causes leukocyte lysis in the lesions of gas gangrene.
Gas Gangrene Morphology of Organism
Clostridium perfringens leads to gas gangrene. Morphology of C. perfringens is:
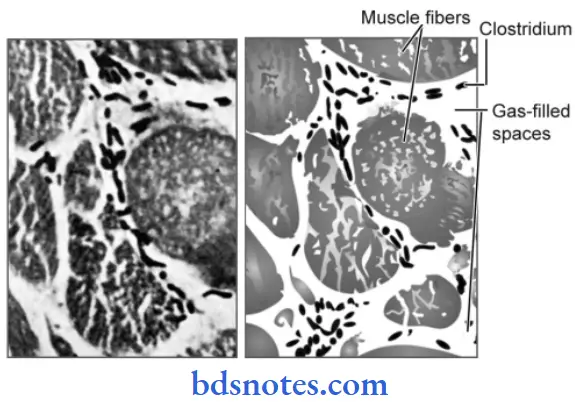
- It is a plump, Gram-positive bacillus with straight, parallel sides, rounded or truncated ends about 4 to 6 microns × 1 micron.
- It may occur singly or in chains.
- It is pleomorphic.
- It is capsulated and non-motile.
- It is filamentous and involution forms are common.
Gas Gangrene Staining Of Organism
C. perfringens is stained by Gram stain and is a Gram-positive organism.
Question 12. Classify Pathological Pigmentation, and Endogenous Pigments In Detail.
Answer:
Pigments are colored substances present in most living beings including humans.
There are two broad categories of pigments:
- Endogenous
- Exogenous.
Pathological Pigments
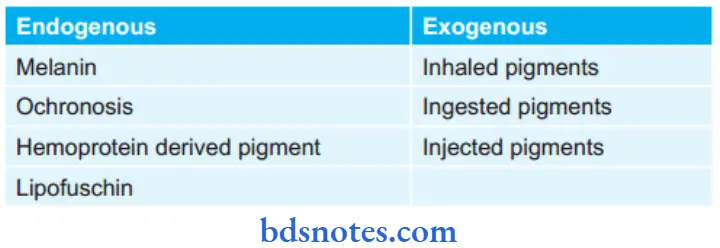
Endogenous Pigments
Endogenous pigments are either normal constituents of cells or accumulate under special circumstances.
Melanin: It is brown black, non-hemoglobin derived pigment.
- It is normally present in hair, skin, choroid of the eye, meninges, and adrenal medulla.
- It is synthesized in melanocyte and dendritic cells.
- It is stored in the form of cytoplasmic granules in the phagocytic cells called melanophores.
- Hyperpigmentation occur during:
- Addison’s disease
- Chronic arsenic poisoning
- Melanosis Coli
- Melanotic tumor
- Hypopigmentation occurs during:
- Albinism
- Leucoderma.
- Vitiligo
- Leprosy
- Radiation dermatitis.
Staining characteristics of melanin: It can be bleached by hydrogen peroxide and is stained with Masson-Fontana argentaff stain, this differentiates the melanin from melanin look-alikes.
Ochronosis: The pigment is melanin-like and is deposited both intracellularly and intercellularly.
- The most commonly affected tissues are cartilage, capsules of joints, ligaments, and tendons.
Hemoprotein Derived Pigment:
- Hemosiderin: It is formed by the aggregation of ferritin.
- It is golden yellow to the brown crystalline, granular pigment that stains with Prussian blue stain.
- It is found within mononuclear phagocytes of bone, spleen, and liver where the breakdown of RBC occurs.
- Severe progressive iron overload leading to fibrosis and organ failure is known as hemochromatosis
- Acid hematin (Hemozoin): It is a hemoprotein-derived brown-black pigment.
- It contains heam iron in the ferric form in an acidic medium.
- It is seen most commonly in chronic malaria and in mismatched blood transfusions.
- Bilirubin: It is an iron-containing pigment present in bile and stain with a gmelin reaction.
- It is derived from the porphyrin ring of hemoglobin.
- Excess bilirubin causes jaundice.
- Porphyrins: They are tetrapyrrole in heam. It contains iron.
- Porphyria results from the genetic deficiency of one of the enzymes required for the synthesis of heam.
Lipofuscin: It is also known as wear and tear pigment.
- Lipofuscin or lipochrome is a yellowish-brown granular intracytoplasmic lipid pigment.
- The pigment is found in atrophied cells of old age.
- It is seen in myocardial fibers, hepatocytes, Leydig cells of the testis, and neurons in senile dementia.
- In heart muscle change is associated with wasting of muscle and is commonly referred to as brown atrophy.
Staining characteristics of lipofuscin
- It is acid-fast
- Autofloroscent
- Stain positive with fat stains
- Reduces ferricyanide to ferrocyanide.
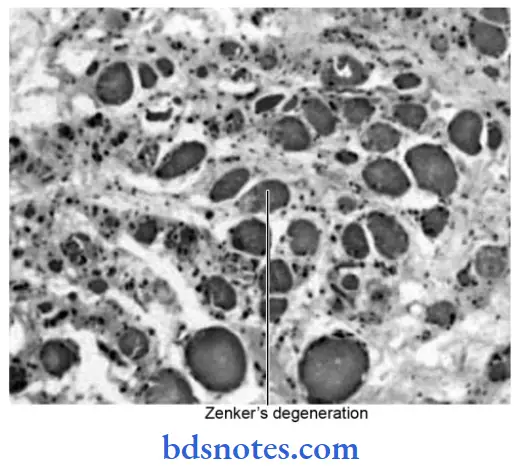
Question 13. Write A Short Note On Zenker’S Degeneration.
Answer:
Zenker’S Degeneration
- Hyaline degeneration of voluntary muscles is also called Zenker’s degeneration.
- It is an intracellular hyaline change associated with heterogeneous pathologic conditions.
- It occurs in the rectus abdominal muscle in typhoid fever.
- The muscle loses its fibrillar staining and becomes glossy and hyaline.
Question 14. Write A Short Note On Apoptosis.
Or
Write In Short About Apoptosis.
Or
Write Note On Apoptosis.
Answer:
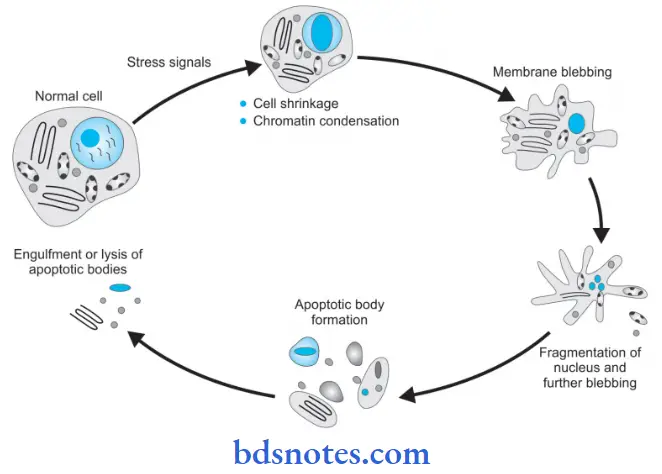
Apoptosis is a form of coordinated and internally programmed cell death.
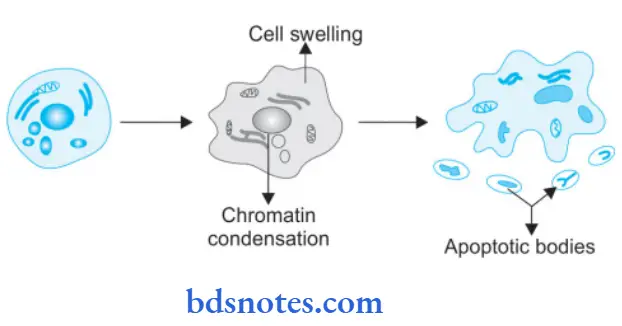
Apoptosis Morphologic Changes
Following are the morphological changes in apoptosis which are seen in an electron microscope:
- Cell shrinkage: The cell is smaller in size; the cytoplasm is dense; and the organelles, although relatively normal, are more tightly packed.
- Chromatin condensation: This is the most characteristic feature of apoptosis. The chromatin aggregates peripherally, under the nuclear membrane, into well-delimited dense masses of various shapes and sizes. The nucleus itself may break up, producing two or more fragments.
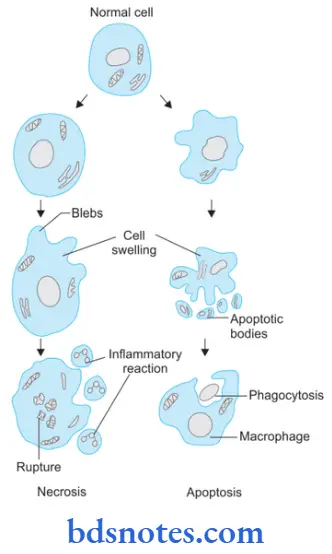
- Formation of cytoplasmic blebs and apoptotic bodies: The apoptotic cell first shows extensive surface blebbing, then undergoes fragmentation into a number of membrane-bound apoptotic bodies composed of cytoplasm and tightly packed organelles, with or without a nuclear fragment.
- Phagocytosis of apoptotic cells or bodies by adjacent healthy cells, either parenchymal cells or macrophages. The apoptotic bodies are rapidly degraded within lysosomes, and the adjacent cells migrate or proliferate to replace the space occupied by the now-deleted apoptotic cell.
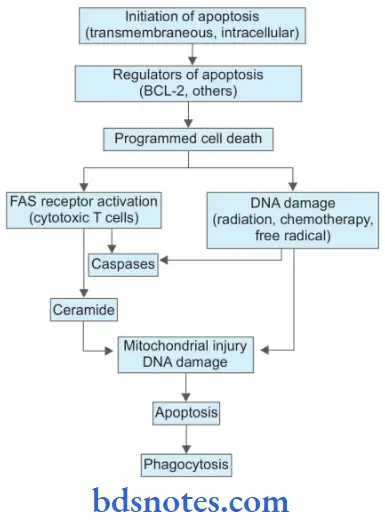
Molecular Mechanism Of Apoptosis
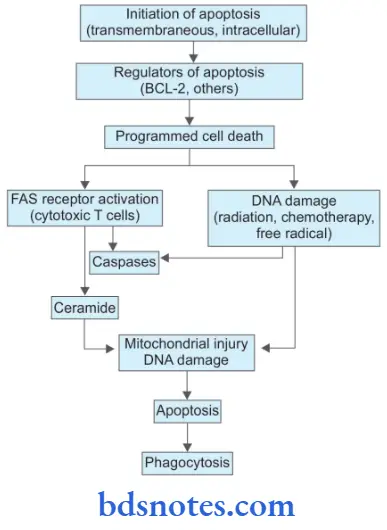
1. Initiators Of Apoptosis
- Stimulation of signaling programmed cell death act either at the cell membrane or intracellularly. This includes:
- Absence of stimuli required for normal cell survival.
- Activators of programmed cell death.
- Intracellular stimuli include heat, radiation, hypoxia, etc.
2. Regulators of apoptosis
- As the cell is involved in apoptosis by above mentioned signals, the next phase is regulation in which certain proteins convert death signals to fail programmed cell death and determine the outcome. Regulator proteins are:
- BCL-2: It is a gene that is located in the outer mitochondrial membrane and may regulate the apoptotic process by binding to some other proteins, For Example. As BCL-2 binds to BAX or BAD it promotes apoptosis while as it binds to BCL-XL it inhibits apoptosis.
- Other apoptotic regulator proteins: Besides BCL-2 other regulatory proteins of apoptosis are TP53 protein, BAX, and certain viruses.
3. Programmed Cell Death
- Final outcome of apoptotic regulators in programmed cell death involves the following pathways:
- FAS receptor activation: Cell surface receptor FAS is present in cytotoxic T cells. On coming in contact with target cells FAS receptor is activated. This activates caspases and leads to proteolysis and further apoptosis.
- Ceramide generation: Due to the hydrolysis of phospholipid, i.e. sphingomyelin of plasma membrane ceramide gets generated. Ceramide is implicated in mitochondrial injury and further apoptosis.
- DNA damage: Damage to DNA by ionizing radiation, chemotherapeutic agents, and activation of oxygen species leads to apoptosis. DNA damage affects TP53 which induces the synthesis of cell death-promoting protein BAX and further apoptosis.
- Phagocytosis: Dead apoptotic cells and their fragments possess cell surface receptors that facilitate their identification by adjacent phagocytes. Phagocytes engulf the apoptotic cells and clear the area.
Diagnosis Of Apoptosis
- Agarose gel electrophoresis shows a step ladder pattern
- Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) technique for in vivo detection
- H&E stain, Feulgen, and acridine orange staining of apoptotic cells
- Measurement of cytosolic cytochrome C and activated caspase
- Expression of phosphatidyl serine on the outer leaflet of the plasma membrane by apoptotic cells enables their recognition by using the dye annexin V.
Disorders Associated With Apoptosis
- Disorders associated with decreased apoptosis, i.e. cancer, autoimmunity
- Disorders associated with increased apoptosis:
- Neurodegenerative diseases, i.e. Alzheimer, Huntington, Parkinson’s disease
- Ischemic injury in stroke and myocardial infarction
- Death of virus—infected cells as in AIDS.
Question 15. Write A Short Note On Pathological Calcification.
Answer:
Pathologic calcification is the abnormal deposition of calcium in various tumors and organs of the body:
They are of three types:
- Dystrophic calcification
- Metastatic calcifiation
- Calcinosis.
Dystrophic Calcification
- It is a type of pathologic calcification in which calcium salts are deposited in the dead or degenerating tissue of the body.
- It is not associated with increased levels of serum calcium and is related to changes in the local environment.
- This is the most frequent type of pathological calcification found in a wide variety of tissues.
- In the mouth area of dystrophic calcification is found in the gingiva, tongue, cheek, and pulp.
- One of the most common intraoral dystrophic calcification found in the pulp of the teeth is “Pulp Stone”.
Metastatic Calcifiation
- Abnormal deposition of calcium in the tissue due to an increase in the amount of serum calcium.
- It occurs particularly in diseases like hyperparathyroidism which depletes the bone calcium and causes high levels of blood calcium.
- Metastatic calcification also occurs in hypervitaminosis D. In this type of calcification deposit of calcium occurs in the kidney, lung, gastric mucosa, and media of blood vessels.
Pathological Calcification Calcinosis
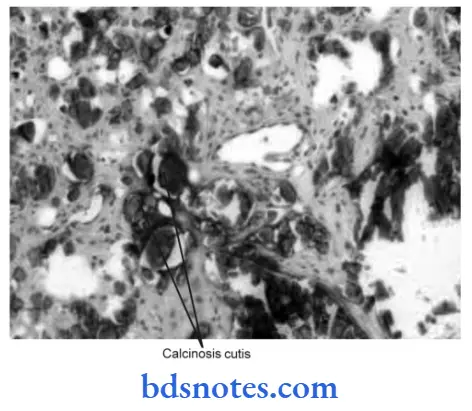
- Abnormal deposition of calcium under the skin is also known as calcinosis.
- There are two forms of calcinosis:
- Calcinosis circumscripta: It is circumscribed form.
- Calcinosis universalis: It is a generalized form and is associated with scleroderma and dermatomyositis.
Gross Pathology Of Pathological Calcification
Pathologic calcification appears as fine white granules or clumps of gritty deposits.
Microscopical Picture Of Pathological Calcification
- In Hematoxylin and Eosin (H&E) stained sections it appears as intracellular or extracellular basophilic amorphous granular deposits.
- At times single necrotic cells act as seeds that get encrusted with lamellar mineral deposits, i.e. psammoma body called so due to its resemblance to grains of sand and commonly seen in some papillary cancers, i.e. thyroid and meningiomas.
- Calcium and iron salts may gather long slender spicules of asbestos in the lungs, creating beaded, dumbbell forms called asbestos bodies.
Question 16. Write A Short Note On Free Radical Injury.
Answer:
Free radicals are chemical species with an unpaired electron in their outer orbit. These free radicals react with both inorganic and organic molecules which are present in membranes and nucleic acids.
Causes Of Free Radical Injury
Free radical generation is induced by
- Absorption of radiant energy, i.e. UV rays, X-rays.
- Enzymatic metabolism of exogenous chemical/drugs, i.e. CCl4 to CCl–3
- Reduction—oxidation reaction processes that occur during normal metabolism, i.e. formation of superoxide anion (O2–). hydrogen peroxide (H2O2) hydroxyl ion (OH–).
- Reactions involving transition metals, i.e. iron (Fenton reaction), copper, etc.
- Reactions involving nitric oxide (NO) which acts as a free radical and can be converted to highly reactive peroxynitrite anion (ONOO– as well as NO2 and NO3–.
Effects Of Free Radical Injury
The hydroxyl radical is the most reactive species. It may produce membrane damage by the following mechanisms:
- Lipid peroxidation: Polyunsaturated fatty acids of the membrane are attacked repeatedly and severely by oxygen-derived free radicals to yield highly destructive polyunsaturated fatty acid radicals, i.e. lipid hydroperoxy radicals and lipid hydroperoxides. This reaction is termed as lipid peroxidation. Lipid peroxidation is propagated to other sites causing widespread membrane damage and destruction of organelles.
- Oxidation of proteins: Oxygen-derived free radicals cause cell injury by oxidation of protein macromolecules of cells, cross-linking of labile amino acids as well as by fragmentation of polypeptides directly. The end result is the degradation of cytosolic neutral proteases and cell destruction.
- DNA damage: Free radicals cause breaks in the single strands of nuclear and mitochondrial DNA. This results in cell injury, and it may also cause malignant transformation of cells.
- Cytoskeletal damage: Reactive oxygen species are also known to interact with cytoskeletal elements and interfere in mitochondrial aerobic phosphorylation and thus cause ATP depletion.
The antioxidants are used to inactivate the free radicals.
Inactivation Of Free Radicals
Inactivation of free radicals is done by:
- Antioxidants, i.e. vitamins A, C, E, and β carotene.
- Iron and copper-binding proteins, i.e. transferrin, ferritin. lactoferrin, ceruloplasmin
- Enzymes such as catalase, superoxide dismutase, and glutathione peroxidase.
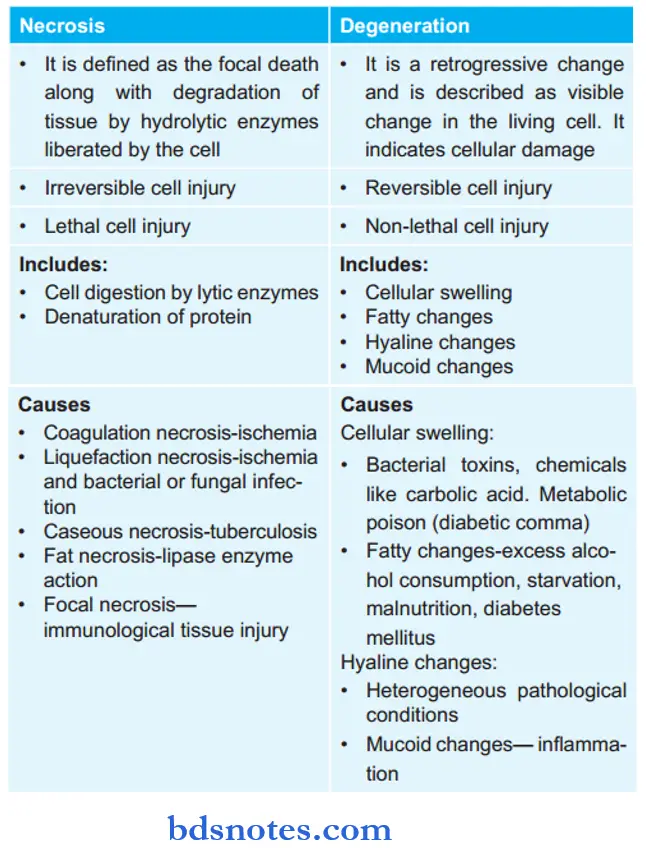
Question 17. Write The Differences Between Necrosis And Degeneration.
Answer:
Question 18. Write The Differences Between Apoptosis And Necrosis.
Answer:
Question 19. Write A Short Note On Gas Gangrene.
Answer:
Gas gangrene is a special form of wet gangrene caused by gas-forming clostridia.
- These bacteria gain entry into the tissues through the open contaminated wound, especially in the muscles, or as a complication of operations on the colon which normally contains clostridia.
- Clostridia produce various toxins which produce necrosis and edema locally and are absorbed producing profound systemic manifestations.
Gas Gangrene Gross Changes
- The affected area is swollen, edematous, painful, and crepitant due to the accumulation of gas bubbles within the tissue.
- The affected tissue becomes dark black and foul-smelling.
Gas Gangrene Microscopically
- Muscle fibers undergo coagulative necrosis with liquefaction.
- A large number of gram-positive bacilli can be identified.
- At the periphery, a zone of leucocyte infiltration, edema, and congestion are found.
- Capillary and venous thrombi are common.
Question 20. Write The Difference Between Moist And Dry Gangrene.
Answer:
Question 21. Define Necrosis. Name Different Types Of Necrosis. What Are Nuclear Changes In Necrosis?
Answer:
Necrosis is defined as focal death along with the degradation of tissue by hydrolytic enzymes liberated by cells.
Types Of Necrosis
There are five types of necrosis:
- Coagulative necrosis
- Liquefaction necrosis
- Caseous necrosis
- Fat necrosis
- Fibrinoid necrosis.
Necrosis Nuclear Changes
The nuclear changes are:
- Pyknosis: Condensation of nuclear chromatin.
- Karyolysis: This may either undergo dissolution.
- Karyorrhexis: Fragmentation into many granular clumps.
Question 22: Define Cell Injury. Write In Brief About Its Pathogenesis.
Answer:
If the limits of adaptive response to a stimulus are exceeded, or in certain instances when adaptation is not possible, a sequence of events follows, loosely termed cell injury.
Cell injury is reversible up to a certain point, but if the stimulus persists or is severe enough from the beginning, the cell reaches the “point of no return” and suffers irreversible cell injury and cell death.
1. Pathogenesis Of Cell Injury
Pathogenesis Of Ischemic And Hypoxic Injury
- Reversible cell injury.
- Irreversible cell injury.
Reversible cell injury
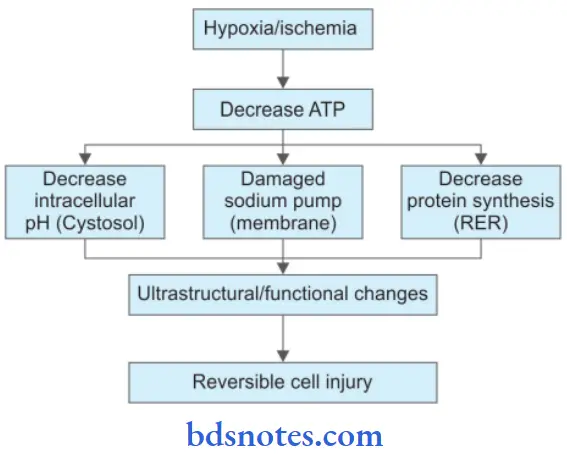
Irreversible Cell Injury
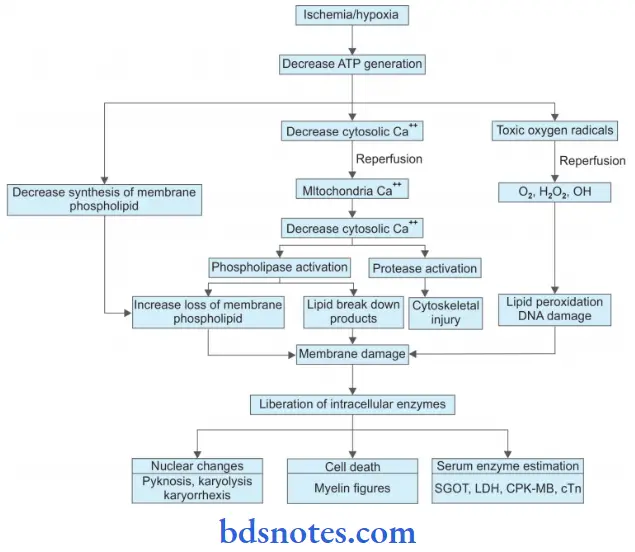
2. Free Radical Mediated Cell Injury
Following is the pathogenesis of free radical-mediated cell injury
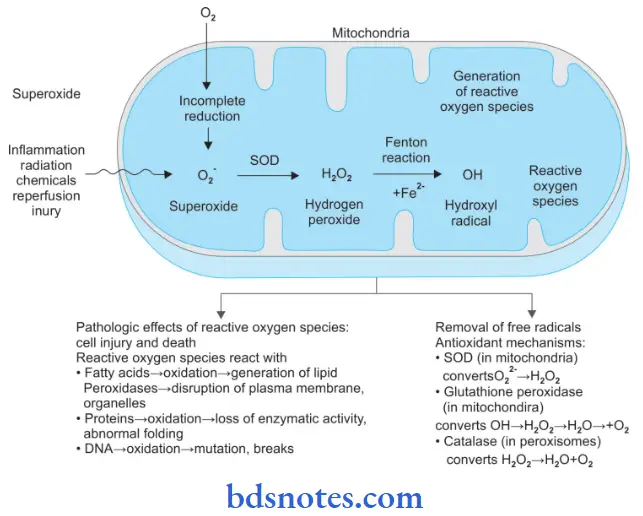
- Generation of oxygen free radicals begins within the mitochondrial inner membrane when cytochrome oxidase catalyzes the four-electron reduction of oxygen (O2) to water (H2O).
Intermediate between the reaction of O2 to H2O, three partially reduced species of oxygen are generated depending upon the number of electrons transferred.
These are:- Superoxide oxygen (O2–): One electron
- Hydrogen peroxide (H2O2): Two electrons
- Hydroxyl radical (OH–): Three electrons.
- A few other oxygen radicals which may be generated in reactions other than those during O2 to H2O are hypochlorous acid (HOCl), peroxynitrite ion (ONOO), nitric oxide (NO) generated by various body cells (endothelial cells, neurons, macrophages, etc.), and release of superoxide free radical in Fenton reaction.
Free Radical Generation
- The three partially reduced intermediate species between O2 to H2O are derived from enzymatic and non-enzymatic reactions as under:
- Superoxide (O2–): Superoxide ion O2– may b generated by direct auto-oxidation of O2 during mitochondrial electron transport reaction. Alternatively O2– is produced enzymatically by xanthine oxidase and cytochrome P450 in the mitochondria or cytosol. O2– so formed is catabolized to produce H2O2 by superoxide dismutase (SOD).
- Hydrogen peroxide (H2O2): H2O2 is reduced to water enzymatically by catalase (in the peroxisomes) and glutathione peroxidase GSH (both in the cytosol and mitochondria).
- Hydroxyl radical (OH–): OH– radical is formed in two ways in biological processes by radiolysis of water and by reaction of H2O2 with ferrous (Fe++) ions; the latter process is termed as Fenton reaction.
Free Radical Reactions
The hydroxyl radical is the most reactive species. Reactive oxygen species (ROS) produce membrane damage by the following mechanisms:
- ROS reacts with fatty acids and leads to oxidation which leads to the generation of lipid peroxidases. This leads to disruption of the plasma membrane and organelles.
- ROS reacts with proteins leading to the oxidation of proteins and there is a loss of enzymatic activity and abnormal folding of proteins.
- ROS reacts with DNA and leads to the oxidation of DNA and there is mutation and breakage in single strands of mitochondrial and nuclear DNA.
3. Cell Injury By Chemicals
Chemicals induce cell injury by one of the following two mechanisms:
- Direct cytotoxic effects: Some chemicals combine with components of the cell and produce direct cytotoxicity without requiring metabolic activation. The cytotoxic damage is usually greatest to cells that are involved in the metabolism of such chemicals. For Example. in mercuric chloride poisoning, the greatest damage occurs to cells of the alimentary tract and kidney, cyanide kills the cell by poisoning mitochondrial cytochrome oxidase thus blocking oxidative phosphorylation.
- Conversion to reactive toxic metabolites: This mechanism involves metabolic activation to yield the ultimate toxin that interacts with the target cells. The target cells in this group of chemicals may not be the same cell that metabolized the toxin. An example of cell injury by the conversion of reactive metabolites is toxic liver necrosis caused by carbon tetrachloride.
4. Cell Injury by Physical Agents/Ionizing Radiation
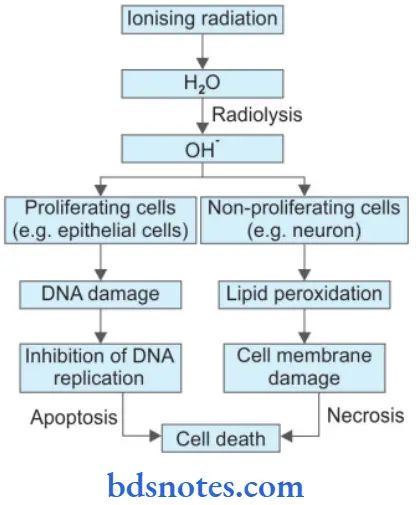
Question 23. Define Cell Injury. Describe Various Causes And Morphological Changes In Cell Injury.
Answer:
If the limits of adaptive response to a stimulus are exceeded, or in certain instances when adaptation is not possible, a sequence of events follows, loosely termed cell injury.
- Cell injury is reversible up to a certain point, but if the stimulus persists or is severe enough from the beginning, the cell reaches the “point of no return” and suffers irreversible cell injury and cell death.
Causes Of Cell Injury
Causes of cell injury are divided into two parts, i.e. genetic and acquired.
Genetic Cause
The genetic injury may result in a defect as gross as the congenital malformations associated with Down syndrome or as subtle as the single amino acid substitution in hemoglobin S in sickle cell anemia.
The many inborn errors of metabolism arising from enzymatic abnormalities, usually an enzyme lack, are excellent examples of cell damage due to subtle alterations at the level of DNA.
Acquired Causes
- Hypoxia and ischemia: Cells of diffrent tissues essentially require oxygen to generate energy and perform metabolic functions. Deficiency of oxygen or hypoxia results in the failure to carry out these activities by the cells.
Hypoxia is the most common cause of cell Injury. The most common mechanism of hypoxic cell injury is by reduced supply of blood to cells, i.e. ischemia. However, oxygen deprivation of tissues may result from other causes as well, For Example. anemia, carbon monoxide poisoning, cardiorespiratory insufficiency, and increased demand for tissues. - Physical agents: Physical agents include mechanical trauma, extremes of temperature (burns and deep cold), sudden changes in atmospheric pressure, radiation, and electric shock.
- Chemicals and drugs: Simple chemicals such as glucose or salt in hypertonic concentrations may cause cell injury directly or by deranging electrolyte homeostasis of cells. Even oxygen, in high concentrations, is severely toxic. Trace amounts of agents known as poisons, such as arsenic, cyanide, or mercuric salts, may destroy sufficient numbers of cells within minutes to hours to cause death. Other substances, however, are our daily companions— environmental and air pollutants, insecticides, and herbicides; industrial and occupational hazards, such as carbon monoxide and asbestos; social stimuli, such as alcohol and narcotic drugs; and the ever-increasing variety of therapeutic drugs.
- Microbial agents: Injuries by microbes include infections caused by bacteria, rickettsiae, viruses, fungi, protozoa, metazoa, and other parasites.
- Immunologic agents: Immunity is a ’double-edged sword’, it protects the host against various injurious agents but it may also turn lethal and cause cell injury, For Example. hypersensitivity reactions, anaphylactic reactions, and autoimmune diseases.
- Nutritional derangements: A deficiency or an excess of nutrients may result in nutritional imbalances. Nutritional deficiency diseases may be due to overall deficiency of nutrients (For Example. starvation), protein-calorie (For Example. marasmus, kwashiorkor), minerals (For Example. anemia), or trace elements. Nutritional excess is a problem in affluent societies resulting in obesity, atherosclerosis, heart disease, and hypertension.
- Psychologic factors: There are no specific biochemical or morphologic changes in common acquired mental diseases due to mental stress, strain, anxiety, overwork, and frustration, For Example. depression, schizophrenia. However, problems of drug addiction, alcoholism, and smoking result in various organic diseases such as liver damage, chronic bronchitis, lung cancer, peptic ulcer, hypertension, ischemic heart disease, etc.
Morphological Changes In Cell Injury
Following are the morphological changes in cell injury:
Reversible Cell Injury
- Cellular swelling is the first manifestation of almost all forms of injury to cells. On microscopic examination, small clear vacuoles may be seen within the cytoplasm; these represent distended and pinched-of segments of the endoplasmic reticulum.
- This pattern of nonlethal injury is sometimes called hydropic change or vacuolar degeneration. The swelling of cells is reversible. The ultrastructural changes of reversible cell injury include:
- Plasma membrane alterations, such as blebbing, blunting, and distortion of microvilli; creation of myelin figures; and loosening of intercellular attachments
- Mitochondrial changes, including swelling, rarefaction, and the appearance of small phospholipid-rich amorphous densities
- Dilation of the endoplasmic reticulum with detachment and disaggregation of polysomes
- Nuclear alterations, with disaggregation of granular and fibrillar elements.
Irreversible Cell Injury
Cell Death or Necrosis
- Necrotic cells show increased eosinophilia.
- The cell may have a more glassy homogeneous appearance than that of normal cells, mainly as a result of the loss of glycogen particles.
- When enzymes have digested the cytoplasmic organelles, the cytoplasm becomes vacuolated and appears moth-eaten.
- Finally, calcification of the dead cells may occur.
- Nuclear changes appear in the form of one of three patterns, all due to nonspecific breakdown of DNA. The basophilia of the chromatin may fade (karyolysis), a change that presumably reflects DNase activity. A second pattern is a pyknosis, characterized by nuclear shrinkage and increased basophilia. Here the DNA apparently condenses into a solid, shrunkenbasophilic mass. In the third pattern, known as karyorrhexis, the pyknotic or partially pyknotic nucleus undergoes fragmentation. With the passage of time (a day or two), the nucleus in the necrotic cell totally disappears.
Programmed Cell Death
The following morphologic features, some best seen with the electron microscope, characterize cells undergoing apoptosis:
- Cell shrinkage: The cell is smaller in size; the cytoplasm is dense; and the organelles, although relatively normal, are more tightly packed.
- Chromatin condensation: This is the most characteristic feature of apoptosis. The chromatin aggregates peripherally, under the nuclear membrane, into well-delimited dense masses of various shapes and sizes. The nucleus itself may break up, producing two or more fragments.
- Formation of cytoplasmic blebs and apoptotic bodies: The apoptotic cell first shows extensive surface blebbing, then undergoes fragmentation into a number of membrane-bound apoptotic bodies composed of cytoplasm and tightly packed organelles, with or without a nuclear fragment.
- Phagocytosis of apoptotic cells or bodies by adjacent healthy cells, either parenchymal cells or macrophages. The apoptotic bodies are rapidly degraded within lysosomes, and the adjacent cells migrate or proliferate to replace the space occupied by the now-deleted apoptotic cell.
Subcellular Alterations In Cell Injury
Various morphologically distinct alterations at sub-cellular levels are seen in acute and chronic forms of cell injury. They are seen at the level of the cytoskeleton, lysosomes, endoplasmic reticulum, and mitochondria.
Cytoskeletal Changes
- Thin filaments: Thin filaments are composed of actin, myosin, and their associated regulatory proteins. Functioning thin filaments are essential for various stages of leukocyte movement or the ability of such cells to perform phagocytosis adequately. Some drugs and toxins target actin filaments and thus affect these processes. For example, cytochalasin B prevents the polymerization of actin filaments, and phalloidin, a toxin of the mushroom Amanita phalloides, binds actin filaments.
- Microtubules: Defects in the organization of microtubules can inhibit sperm motility, causing male sterility, and at the same time can immobilize the cilia of respiratory epithelium, causing interference with the ability of
this epithelium to clear inhaled bacteria, leading to bronchiectasis. - Intermediate filaments: These components provide a flexible intracellular scaffold that organizes the cytoplasm and resists forces applied to the cell. Various classes of intermediate filaments may deposit in the cytosol.
Lysosomal Changes
- Lysosomeshavepowerfulhydrolyticenzymes. Heterophagy and autophagy are two ways by which lysosomes show morphologic changes in phagocytic function.
- Heterophagy: Phagocytosis, i.e. cell eating, and pinocytosis, i.e. cell drinking are two forms of heterophagy by which material from outside is taken up by lysosomes of polymorphs and macrophages to form phagolysosomes.
- Autophagy: It is the process by which worn-out intracellular organelles and other cytoplasmic material form an autophagic vacuole that fuses with the lysosome to form autophagy so.
Smooth Endoplasmic Reticulum Changes
Hypertrophy of the smooth endoplasmic reticulum occurs, for example, hypertrophy of the smooth endoplasmic reticulum of liver cells occurs as an adaptive change in response to prolonged use of barbiturates.
Mitochondrial Changes
Morphological changes seen in mitochondria are:
- Megamitochondria: It is large mitochondria seen in alcoholic liver disease
- Alteration in the number of mitochondria: Number increases in hypertrophy and decreases in atrophy
- Myopathies are the defects in which mitochondria have abnormal cristae.
Question 22. Write A Short Note On Metastatic Calcification.
Answer:
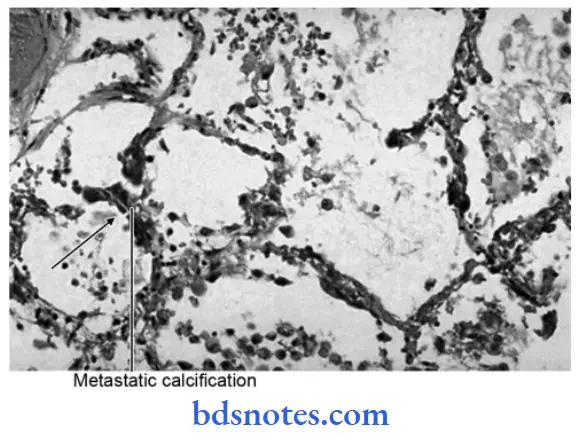
The deposition of calcium salts in vital tissues is known as metastatic calcification.
- It always reflects some disturbance in calcium metabolism, leading to hypercalcemia.
- Abnormal deposition of calcium in the tissue due to an increase in the amount of serum calcium.
Metastatic Calcification Pathogenesis
This is favored by relatively high pH at certain sites, i.e. lung, stomach, blood vessel, and cornea.
Metastatic Calcification Etiology
The causes of metastatic calcification include one of the following two conditions:
- Excessive mobilization of calcium from bones.
- Excessive absorption of calcium from the gut.
Excessive Mobilization Of Calcium From Bones
- It occurs particularly in diseases like hyperparathyroidism which depletes bone calcium and causes high levels of blood calcium
- Bony destructive lesions such as multiple myeloma and metastatic carcinoma.
Excessive Absorption Of Calcium From Gut
- Metastatic calcifiation also occurs in hypervitaminosis D
- Milk-alkali syndrome by excessive oral intake of calcium
- Hypercalcemia of infancy.
Question 23. Write Briefl On Wet Gangrene.
Answer:
It occurs in moist tissues such as the mouth, bowel, lung, cervix, vulva, etc.
- Wet gangrene develops rapidly due to blockage of venous and less commonly arterial blood flow from thrombosis or embolism.
- The affected part gets stuffed with blood which favors the rapid growth of putrefactive bacteria.
- Examples of wet gangrene are diabetic foot which occurs due to high sugar content and favors the growth of bacteria, and bed sores in bedridden patients which lead to the growth of bacteria.
Wet Gangrene Pathology
Macroscopically: Affected part is soft, swollen, putrid, rotten, and dark.
Wet Gangrene Microscopically
- Presence of coagulative necrosis with the stuff of the affected part with blood.
- Mucosa is ulcerated and sloughed.
- There is the presence of intense acute inflammatory exudates and thrombosed vessels.
- The line of demarcation between the gangrenous segment and viable bowel is not clear cut.
Question 24. Write A Short Note On Liquefaction Necrosis.
Answer:
It is also known as colliquative necrosis
- When necrosed cells and tissues are converted into structure-less flids it is called liquefactive necrosis.
- It occurs commonly due to ischemia and bacterial or fungal infections, due to the degradation of tissues by the action of powerful hydrolytic enzymes.
- The cellular structure of the organ is lost and the tissue is digested, it is converted into a liquefied mass which appears creamy yellow in color and is called pus.
- The common examples are infarct brain and abscess cavity.
Liquefaction Necrosis Mechanism
Liquefaction Necrosis Gross Features
- Nerve tissues become liquefied after necrosis because it contains more lipid and water which do not coagulate.
- The affected area is set with a liquefied center containing necrotic debris.
Liquefaction Necrosis Microscopic Features
- Microscopically liquefied tissue has no structure. The cystic space contains necrotic debris and phagocytosed material and macrophage.
- The cyst wall is formed by the proliferation of capillaries, inflammatory cells, and glial cells in case of the brain and proliferating fibroblasts in the case of the abscess cavity.
Question 25. Write A short note on hemosiderin.
Answer:
It is a hemoprotein-derived pigment.
- It is formed by the aggregates of ferritin and is identified in light microscopy.
- It is a golden yellow to brown, granular pigment especially within mononuclear phagocytes of bone marrow, spleen, and liver.
- Hemosiderin is a ferric iron which is demonstrated by the Prussian blue reaction.
- Excessive storage of hemosiderin occurs in a situation where there is an increased breakdown of red cells or systemic overload of iron due to primary hemochromatosis and secondary causes such as thalassemia, sideroblastic anemia, and alcoholic cirrhosis.
- Effects of hemosiderin excess are:
- Localized: It develops due to hemorrhage in local tissues. With the lysis of red cells, hemoglobin is liberated and is taken up by macrophages and is degraded and stored as hemosiderin, For Example. hemorrhage in tissues, black eye, infarction, brown induration of lung.
- Systemic: Systemic overload of iron leads to generalized hemosiderosis. It is of two types:
- Parenchymal deposition in the liver, pancreas, kidney, heart, and skin.
- Reticuloendothelial cell deposits in the liver, spleen, and bone marrow
Following are the examples of systemic hemosiderosis:
- Acquired hemosiderosis in chronic hemolytic disorders, blood transfusion, parenteral administration of iron.
- Hereditary hemochromatosis
- Excessive dietary intake, i.e. in Bantu’s disease.
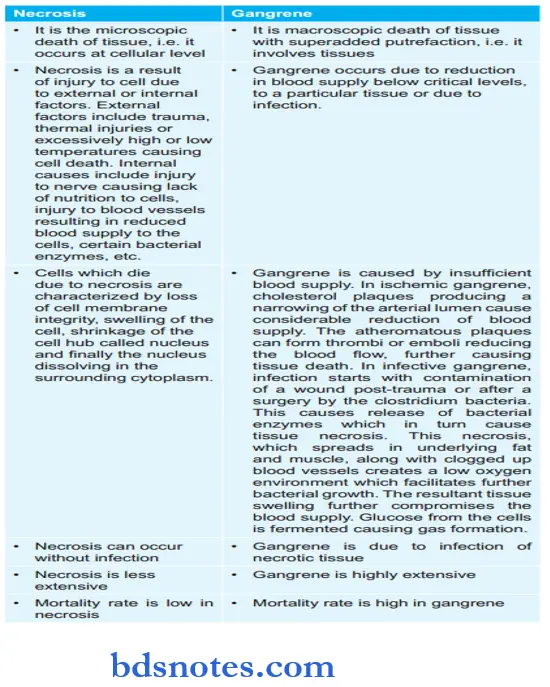
Question 27. Classify Necrosis. Give Differences Between Necrosis And Gangrene.
Answer:
Classification of Necrosis
- Coagulative Necrosis
- Liquefaction Necrosis
- Caseous Necrosis
- Fat Necrosis
- Fibrinoid Necrosis.
Differences Between Necrosis And Gangrene
Question 28. Define Necrosis And Enumerate The Causes Of Necrosis. Write The Pathogenesis Of Caseous Necrosis.
Answer:
Necrosis is defined as focal death along with the degradation of tissue by hydrolytic enzymes liberated by cells.
Causes Of Necrosis
- Hypoxia
- Physical agents: Physical agents include mechanical trauma, extremes of temperature (burns and deep cold), sudden changes in atmospheric pressure, radiation, and electric shock.
- Chemicals and drugs: Trace amounts of agents known as poisons, such as arsenic, cyanide, or mercuric salts, may destroy sufficient numbers of cells within minutes to hours to cause death.
- Microbial agents: Injuries by microbes include infections caused by bacteria, rickettsiae, viruses, fungi, protozoa, metazoa, and other parasites.
- Immunologic agents: Hypersensitivity reactions, anaphylactic reactions, and autoimmune diseases.
Pathogenesis Of Caseous Necrosis
Caseous necrosis is seen in tuberculosis. The development of caseous necrosis is possibly due to the interaction of mycobacteria with activated T cells as well as by the direct toxicity of mycobacteria on macrophages.
Question 29. Write Briefl On AntiOxidants.
Answer:
Anti-oxidants are also known as free radical scavengers.
- An antioxidant is a molecule that inhibits the oxidation of other molecules. Oxidation is a chemical reaction that can produce free radicals, leading to chain reactions that may damage cells. Antioxidants terminate these chain reactions.
- The body makes some of the antioxidants it uses to neutralize free radicals. These antioxidants are called endogenous antioxidants. However, the body relies on external (exogenous) sources, primarily the diet, to obtain the rest of the antioxidants it needs. These exogenous antioxidants are commonly called dietary antioxidants.
- The anti-oxidants are:
- Vitamins A, E, and C
- Sulfhydryl-containing compounds For Example. cysteine and glutathione
- Serum proteins, For Example. ceruloplasmin and transferrin.
Antioxidants may play a role in the management or prevention of some medical conditions, such as some cancers, macular degeneration, Alzheimer’s disease, and some arthritis-related conditions.
Question 30. Write Short Note On Hypertrophy.
Or
Write A Note On Hypertrophy.
Answer:
Hypertrophy refers to an increase in the size of cells and, with such change, an increase in the size of the organ. Thus, the hypertrophied organ has no new cells, just larger cells.
- The increased size of the cells is not due to cellular swelling but due to the synthesis of more structural components.
Hypertrophy Types
Hypertrophy can be physiologic or pathologic and is caused by increased functional demand or by specific hormonal stimulation.
- Physiologic hypertrophy: The massive physiologic growth of the uterus during pregnancy is a good example of hormone-induced hypertrophy involving both hypertrophy and hyperplasia. Cellular hypertrophy is stimulated by estrogenic hormones through smooth muscle estrogen receptors, which allow for interactions of the hormones with nuclear DNA, eventually resulting in increased synthesis of smooth muscle proteins and an increase in cell size.
Similarly, prolactin and estrogen cause hypertrophy of the breasts during lactation. These are examples of physiologic hypertrophy affected by hormonal stimulation. - Pathologic hypertrophy: Various diseases associated with hypertrophy are:
- Hypertrophy of cardiac muscle: It can be seen in various cardiovascular diseases. A few conditions producing left ventricular hypertrophy are mitral insufficiency, aortic valve disease, and systemic hypertension.
- Hypertrophy of smooth muscles: It is seen in cardiac achalasia, pyloric stenosis, intestinal strictures, and muscular arteries in hypertension.
- Hypertrophy of skeletal muscles: It is seen in hypertrophied muscles in athletes and laborers.
- Compensatory hypertrophy: It is seen in the organ where the contralateral organ is removed, i.e.
- Following a nephrectomy on one side of a young patient compensatory hypertrophy occur in the nephrons of the other kidney.
- Adrenal hyperplasia following removal of one adrenal gland.
Question 31. Write A Short Note On Hyperplasia.
Answer:
Hyperplasia constitutes an increase in the number of parenchymal cells in an organ or tissue, which may then have increased volume.
- Hyperplasia takes place if the cellular population is capable of synthesizing DNA, thus permitting mitotic division.
- Labile cells, i.e. epithelial cells of skin and mucus membrane; cells of bone marrow and lymph nodes and stable cells i.e. cells of the liver, pancreas, and kidney can undergo hyperplasia.
- Hyperplasia can be physiologic or pathologic.
Physiologic Hyperplasia
1. Hormonal hyperplasia: Hyperplasia undergoing the influence of hormonal stimulation, For Example. hyperplasia of the female breast during pregnancy and puberty, hyperplasia of the pregnant uterus, and prostatic hyperplasia in old age.
2. Compensatory hyperplasia: It occurs following the removal of an organ or contra-angle organ in paired organs, For Example. regeneration of the liver following partial hepatectomy, nephrectomy of one kidney leads to hyperplasia of nephrons of the contralateral kidney.
Pathologic Hyperplasia
Most forms of pathologic hyperplasia are instances of excessive hormonal stimulation or are the effects of growth factors on target cells, For Example. endometrial hyperplasia due to excess estrogen, pseudo carcinomatous hyperplasia of skin.

Leave a Reply