Enzymes
Question 1. Classification of enzymes with suitable examples.
Answer.
Definition Of Enzymes
Enzymes may be defined as biocatalysts synthesized by living cells. They are protein in nature, colloidal, thermo labile in character and specific in action
Classification Of Enzymes
- In order to have uniformity in identification of enzymes, the International Union of Biochemistry (IUB) adopted a nomenclature system based on chemical reaction type and reaction mechanism
- According to this system the enzymes are grouped in six classes
- The enzymes are classified as
- Oxidoreductases
- Transferases
- Hydrolases
- Lyases
- Isomerases
- Ligases
Oxidoreductases
- These enzymes are involved in oxidation reduction reactions
- The reaction is

- Examples of oxidoreductases:
- Alcohol dehydrogenase
- Acyl CoA dehydrogenase
- Glyceraldehyde 3 phosphate dehydrogenase
- Cytochrome oxidase
- Amino acid oxidase
- Xanthine oxidase
- Glutathione reductase
- Catalase
Transferases
- Enzyme that catalyze the transfer of a particular group from one substrate to another
- The reaction is

- Examples of transferases are:
- Aspartate transaminase
- Ornithine carbamoyl transferase
- Methyl transferase
- Phosphorylase
- Hexokinase
Hydrolases
- These are enzymes which bring about hydrolysis of various compounds
- The reaction is
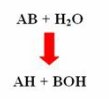
- Examples of hydrolases are:
- Alkaline phosphatase
- Glucose 6 phosphatase
- Lipase
- Choline esterase
- Urease
- Amylase
- Pepsin
- Trypsin
Lyases
- Enzymes that help in the removal of a small molecule from a large substrate
- The reaction is
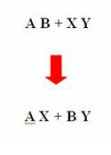
- Examples of lyases are:
- Histidase
- Argininosuccinase
- Aldolase
- Fumarase
Isomerases
- These are enzymes involved in isomerization reactions
- The reaction is
A—> A’ - Examples of isomerases are:
- Phospho triose isomerase
- Retinol isomerase
- Maleyl acetoacetate isomerase
- UDPG epimerase
- Racemase
Ligases
- These are enzymes involved in joining together of 2 substrates
- The reaction is

- Examples of ligases are:
- Glutamine synthetase
- Glycogen synthetase
- Delta ALA synthase
- Acetyl CoA carboxylase
- Succinate thiokinase
- DNA ligase
Question 2. Define enzymes. Explain in detail factors affecting enzyme action.
Answer.
Definition Of Enzymes
Enzymes may be defined as biocatalysts synthesized by living cells. They are protein in nature, colloidal, thermo labile in character and specific in action
Factors Affecting Enzyme Action
The factors which affect enzyme action are:
- Temperature
- pH
- Enzyme concentration
- Product concentration
- Substrate concentration
- Coenzymes
- Metal ion activators
- Inhibitors
Effect Of Temperature
- Each enzyme is most active at a particular temperature known as optimum temperature
- Optimum temperature of most enzymes is in the range of 35 to 40 degrees Celsius
- Reaction velocity almost doubles with 10 degrees Celsius rise in temperature in many enzymes but a very great increase in temperature can cause denaturation of enzyme
- Activity of the enzyme progressively decreases when the temperature of the reaction is below or above the optimum temperature
- It shows a bell shaped curve
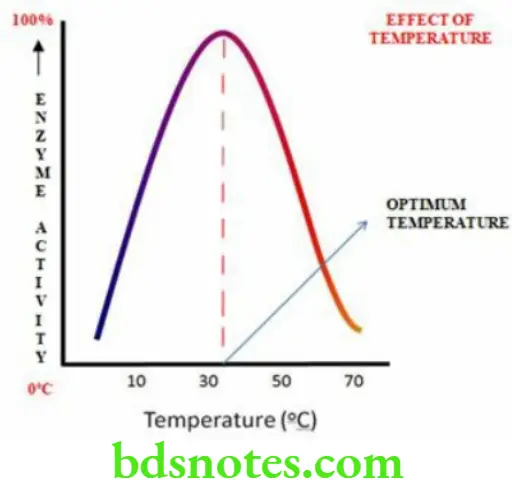
Effect Of pH
- The enzymatic activity is optimum at a particular pH known as optimum pH
- The optimum pH for most enzymes is in the range of 4 to 9
- At a very low or very high pH the enzyme structure may be destroyed
- It shows a bell shaped curve
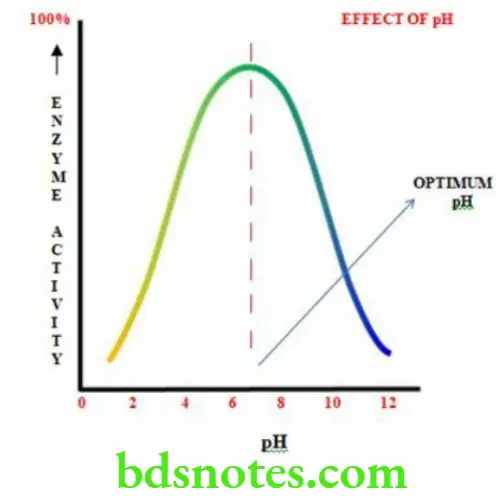
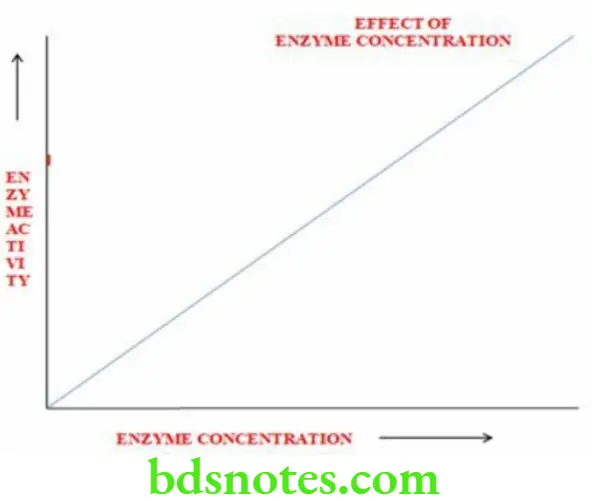
Effect Of Enzyme Concentration
- Velocity of the enzymatic reaction is directly proportional to the enzyme concentration
- Providing more enzyme molecules enables the conversion of progressively larger number of substrate molecules into products
Effect Of Product Concentration
- Products formed as a result of reaction may accumulate and this excess of product may lower the reaction by occupying the active site of the enzyme
- In certain conditions a high concentration of products may cause a reverse reaction forming back the substrate.
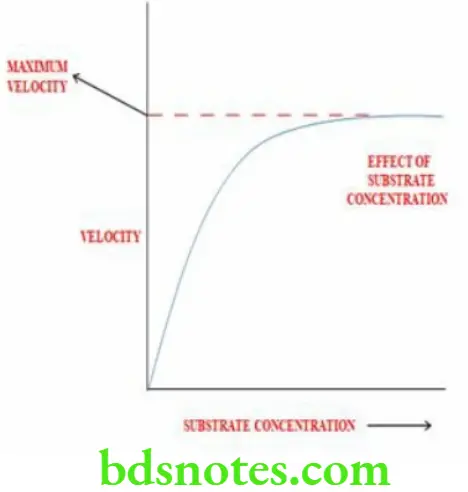
Effect Of Substrate Concentration
- For a known quantity of enzyme, the reaction is directly proportional to the substrate concentration.
- However this is true only upto a certain concentration after which the increasing concentration of substrate does not further increase the velocity of the reaction
Effect Of Coenzyme
- Some enzymes require non protein, organic, low molecular weight substances known as coenzymes for their action
- These enzymes cannot act in the absence of coenzymes
- Examples –
- TPP is the coenzyme for transketolase enzyme.
- NAD is the coenzyme for lactate dehydrogenase.
Effect Of Metal Ion Activators
- Some enzymes require certain inorganic metallic cations for their optimum activity.
- Examples
- ATPase requires calcium
- Enolase requires magnesium
- Phenol oxidase requires copper
- Pyruvate oxidase requires manganese
- Xanthine oxidase requires molybdenum
- Cytochrome oxidase requires iron
Effect Of Enzyme Inhibitors
- Enzyme inhibitor is defined as a substance which binds with the enzyme and brings about a decrease in the enzyme activity.
- Examples –
- Lactate dehydrogenase is inhibited by oxamate
- HMG COA reductase is inhibited by HMG
- Aconitase is inhibited by transaconitate
- Succinate dehydrogenase is inhibited by malonate.
Read And Learn More: BSc Nursing 1st Year Nutrition And Biochemistry Previous year Question and Answers
Question 3. Describe competitive and non- competitive inhibition of enzymes with their examples.
Answer.
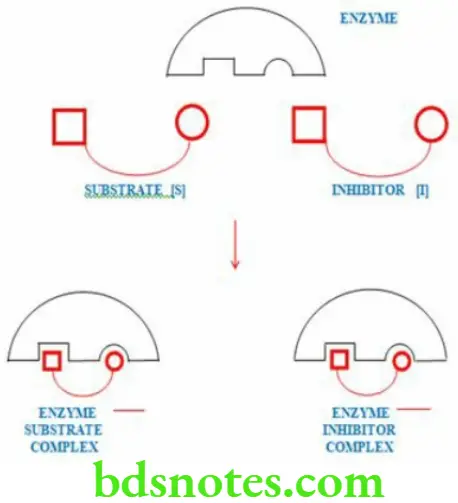
Competitive Inhibiton
- When the active site of the enzyme is occupied by a substance other than the substrate of the enzyme, its activity is inhibited.
- This is known as competitive inhibition
- It is a reversible type of inhibition
- In competitive inhibition both ES and EI complexes are formed during the reaction
- The affinity of the substrate for the enzyme is progressively decreased with the increase in concentration of the inhibitor, thus lowering the rate of enzymatic reaction
- However, when the concentration of substrate is increased, the effect of inhibitor can be reversed, forcing it out of the EI complex
Clinical Importance Of Competitive Inhibitors
- Allopurinol
- Enzyme – Xanthine oxidase
- Substrate – Xanthine
- Inhibitor – Allopurinol
- Significance – Treatment of gout
- Amphetamine
- Enzyme – Monoamine oxidase
- Substrate – Epinephrine
- Inhibitor – Amphetamine
- Significance – To elevate catecholamine levels
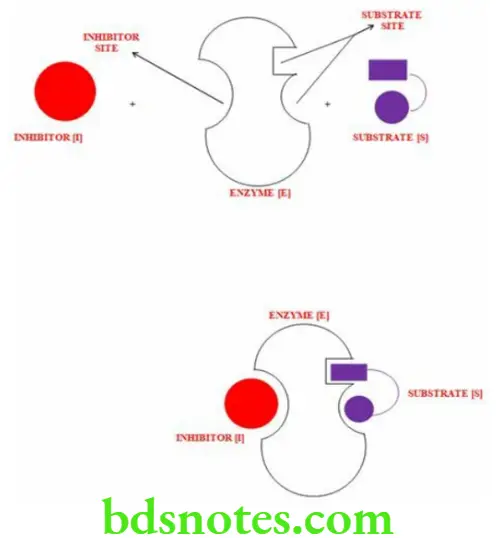
Non Competitive Inhibition
- This occurs when substances not resembling geometry of the substrate do not exhibit mutual competition.
- The sites of attachment of the substrate and inhibitor are different
- The inhibitor binds with a site on the enzyme other than the active site.
- The inhibitor may combine with both free enzyme and ES complex.
- There are 2 types of non competitive inhibition –
- Reversible
- Irreversible
- Reversible non competitive inhibition
- If the inhibitor can be removed from its site of binding without affecting the activity of the enzyme, it is called reversible non competitive inhibition.
- Example – heavy metal ions (lead, silver, mercury) inhibit enzymes by binding with the cysteinyl sulfhydryl groups.
- Irreversible non competitive inhibition
- If the inhibitor can be removed from the site of binding only with the loss of enzymatic activity, it is called irreversible non competitive inhibition
- Example – iodoacetate inhibits the enzyme glyceraldehyde 3 phosphate dehydrogenase

Leave a Reply