Composition And Metabolism Of Amino Acids And Proteins
Question 1. Define proteins, classify them giving suitable examples.
Answer.
Proteins Definition
Proteins are polymers of amino acids.
They are the fundamental structural components of the body
Classification Of Proteins
Proteins can be classified in four ways
- Classification based on shape and size
- Functional classification
- Classification based on chemical nature and solubility
- Nutritional classification
Classification On The Basis Of Shape And Size
On the basis of shape and size proteins are classified into 2 types – fibrous and globular
Fibrous Proteins
When the axial ratio of length to width of a protein molecule is more than 10, it is called a fibrous protein eg – keratin and collagen
Globular Proteins
When the axial ratio of length to width of a protein molecule is less than 10, it is called globular protein eg – haemoglobin and ribonuclease
Functional Classification Of Proteins
Based on the functions they perform, proteins are classified as
- Structural proteins – they are involved in formation of structures of the body e.g. – keratin of hair and nail and collagen of bone
- Enzyme proteins – all enzymes are protein in nature e.g. – hexokinase, pepsin
- Transport proteins – proteins involved in transport of substances e.g. –
- Haemoglobin transports oxygen
- Albumin transports bilirubin
- Hormonal proteins- some of the hormones are protein in nature e.g. Insulin and growth hormone
- Contractile proteins – proteins which take part in muscle contraction. e.g. – Actin and myosin
- Storage proteins – proteins involved in storage of substances e.g. – Ferritin stores iron
- Genetic proteins – proteins involved in genetic function e.g. – Nucleoprotein
- Defence proteins – proteins involved in defence function e.g. – Immunoglobulins
- Receptor proteins – protein which act as receptors e.g. – Cytokine receptor, integrin
- Respiratory proteins – proteins involved in the function of respiration e.g. – Haemoglobin and cytochrome
Classification Based On Chemical Nature And Solubility
According to this proteins are classified into 3 groups – simple, conjugated and derived
Simple Proteins
These are proteins which on complete hydrolysis yield only amino acids.
Example:
- Protamine – they are small molecules rich in arginine
- Histones – they are found in association with DNA
- Albumin – normal serum level is 3.5 to 5 gm %
- Globulin – normal serum level is 1.8 to 3.6 gm %
- Gliadin – it is rich in proline
Nutritional Classification Of Proteins
From the nutritional point of view proteins are classified as
- Complete proteins
- Partially incomplete proteins
- Incomplete proteins
Complete Proteins
These proteins have all the essential amino acids in the required proportions by the human body to promote good growth e.g. egg albumin and milk casein
Partially Incomplete Protein
These proteins are partially lacking one or more essential amino acids and hence can promote moderate growth e.g. – wheat and rice proteins (lack lysine and threonine)
Incomplete Proteins
These proteins completely lack one or more essential amino acids, hence do not promote growth at all e.g. – gelatin (lacks tryptophan), maize/corn (lacks tryptophan and lysine)
Question 2. Transamination reactions.
Answer.
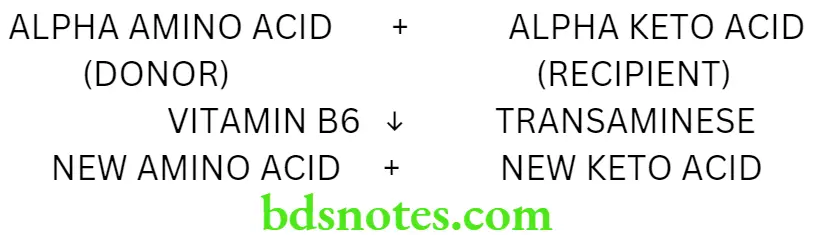
Transamination Definition
Transamination is a reaction in which the amino group of one amino acid is transferred to a keto acid, resulting in the formation of a new amino acid and a new keto acid
Characteristic Features
- Transamination is a reversible reaction
- It takes place in the liver, kidney, heart and brain
- The enzyme required is called transaminase
- Pyridoxine is required as a coenzyme for the reaction
- While most amino acids can act as donors, the recipient keto acids may be alpha ketoglutarate, oxaloacetate or pyruvate
- All the recipient keto acids are components of the Krebs cycle
- The amino acids which do not take part in transamination are lysine, threonine, proline and hydroxyproline
Question 3. Urea Cycle.
Answer.
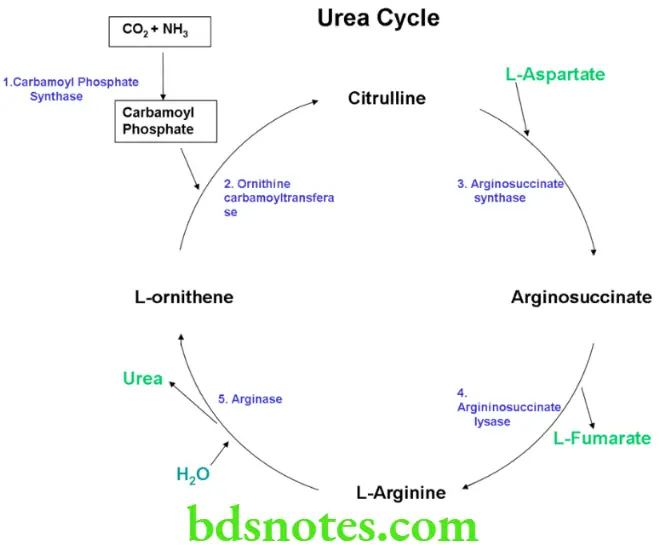
Urea Cycle Definition
The removal of excess ammonia derived from amino acid catabolism is accomplished by the production of urea, which is excreted in the urine.
This cycle is known as urea cycle
Characteristic Features
- It is also known as Krebs-Henseleit cycle
- Urea cycle takes place in the liver, kidney, intestine and brain.
- Urea synthesis does not occur in the brain, kidney or intestine due to absence of some enzmyes
- Urea formation takes place only in the liver
- Urea cycle takes place partly in the mitochondria and partly in the cytoplasm
- One molecule of ammonia and one molecule of carbon dioxide are converted to one molecule of urea in each turn of the cycle and ornithine is regenerated at the end
- Each turn of the urea cycle requires 4 ATP’s.
Importance Of Urea Cycle:
- The major biological role of urea cycle is detoxification of ammonia
- Toxic ammonia is converted to non toxic urea and excreted in the urine
- The normal blood ammonia level is 40 to 70 micrograms %
- Hyperammonemia occurs due to cirrhosis of the liver or genetic defects in urea cycle enzymes.
Read And Learn More: BSc Nursing 1st Year Nutrition And Biochemistry Previous year Question and Answers
Question 4. Write a note on transamination and deamination reactions in protein metabolism.
Answer.
Transamination Definition
Transamination is a reaction in which the amino group of one amino acid is transferred to a keto acid, resulting in the formation of a new amino acid and a new keto acid
Characteristic Features Of Transamination
- Transamination is a reversible reaction
- It takes place in the liver, kidney, heart and brain
- The enzyme required is called transaminase
- Pyridoxine is required as a coenzyme for the reaction
- While most amino acids can act as donors, the recipient keto acids may be alpha ketoglutarate, oxaloacetate or pyruvate
- All the recipient keto acids are components of the Krebs cycle
- The amino acids which do not take part in transamination are lysine, threonine, proline and hydroxyproline.

Deamination Definition
The removal of amino group from the amino acid is known as deamination
Types Of Deamination
It can be of two types
- Oxidative deamination
- Non oxidative deamination
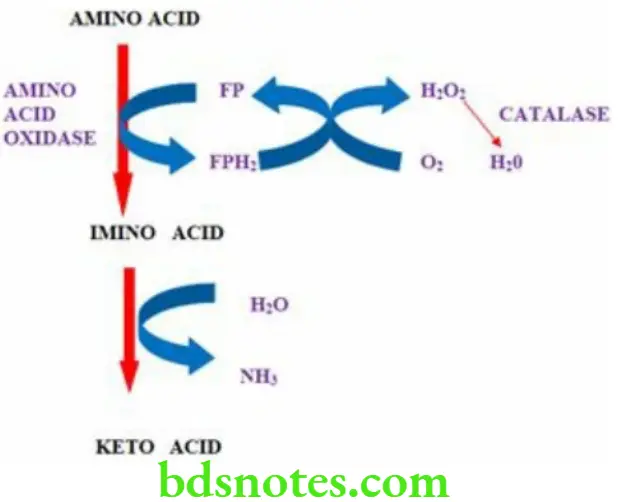
Oxidative Deamination
- It is a process in which ammonia is removed from the amino acid
- It takes place in the liver and kidney
- The enzyme required is amino acid oxidase
- Amino acid is converted to imino acid which then forms keto acid and ammonia is eliminated
- Hydrogen peroxide is formed, which is toxic to the cells and is immediately broken down by the enzyme catalase
- It does not play a major role in the formation of ammonia.
Question 5. Define oxidative and non- oxidative deamination.
Answer.
Deamination Definition
The removal of amino group from the amino acid is known as deamination
Types Of Deamination
It can be of two types
- Oxidative deamination
- Non oxidative deamination
Oxidative Deamination
- It is a process in which ammonia is removed from the amino acid
- It takes place in the liver and kidney
- The enzyme required is amino acid oxidase
- Amino acid is converted to imino acid which then forms keto acid and ammonia is eliminated
- Hydrogen peroxide is formed, which is toxic to the cells and is immediately broken down by the enzyme catalase
- It does not play a major role in the formation of ammonia.

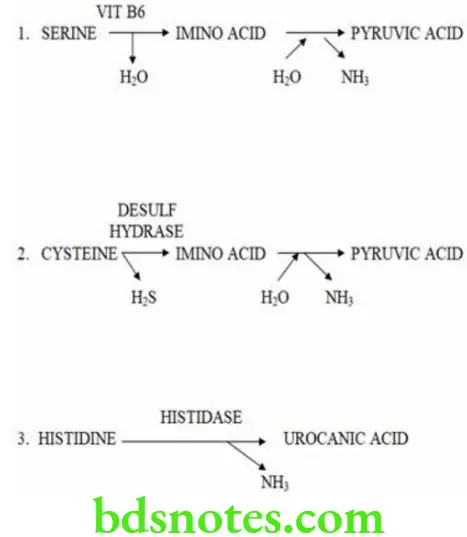
Non Oxidative Deamination
- Certain amino acids can be non-oxidatively deaminated by specific enzymes to form ammonia
- It does not play a major role in ammonia formation.

Leave a Reply