Antihyperlipidemic Agents Introduction
Hyperlipidemia is the most prevalent indicator of susceptibility to atherosclerotic heart disease; it is a term used to describe elevated plasma levels of lipids that are usually in the form of lipoproteins.
- Lipoproteins are macromolecules consisting of lipid substances (cholesterol, triglycerides) non-covalently bound with protein and carbohydrates.
- These combinations solubilize the lipids and prevent them from forming insoluble aggregates in the plasma. Each lipoprotein is associated with an additional protein known as apolipoproteins.
- The various lipoproteins found in plasma can be separated by ultra-centrifugal techniques into chylomicrons, very-low-density lipoprotein (VLDL), intermediate-density lipoprotein low-density lipoprotein (LDL), and high-density lipoprotein (HDL).
- The excess plasma concentration of one or more of these compounds is known as hyperlipidemia.
- Because all lipids require the presence of soluble lipoproteins to be transported in the blood, hyperlipidemia ultimately increases the concentration of these transport molecules.
A condition known as hyperlipoproteinemia and it is strongly associated with atherosclerotic lesions and coronary heart disease.
Read and Learn More Medicinal Chemistry II Notes
Lipoprotein Metabolism
The rate at which cholesterol and triglycerides enter the circulation from the liver and small intestine depends on the supply of the lipids and proteins necessary to form the lipoprotein complexes.
- Although the protein component must be synthesized, the lipids can be obtained either from de novo biosynthesis in the tissues or from the diet. Reduction of plasma lipids by diet can delay the development of atherosclerosis.
- Furthermore, the use of drugs that decrease assimilation of lipids into the body plus diet decreases mortality from cardiovascular disease.
- The lipid transport mechanisms that exist shuttle cholesterol and triglycerides among the liver, intestine, and other tissues.
- Normally, plasma lipids, including lipoprotein cholesterol, are cycled in and out of plasma and do not cause extensive accumulation of deposits in the walls of arteries.
- Genetic factors and changes in hormone levels affect lipid transport by altering enzyme concentrations and apoprotein content, as well as the number and activity of lipoprotein receptors.
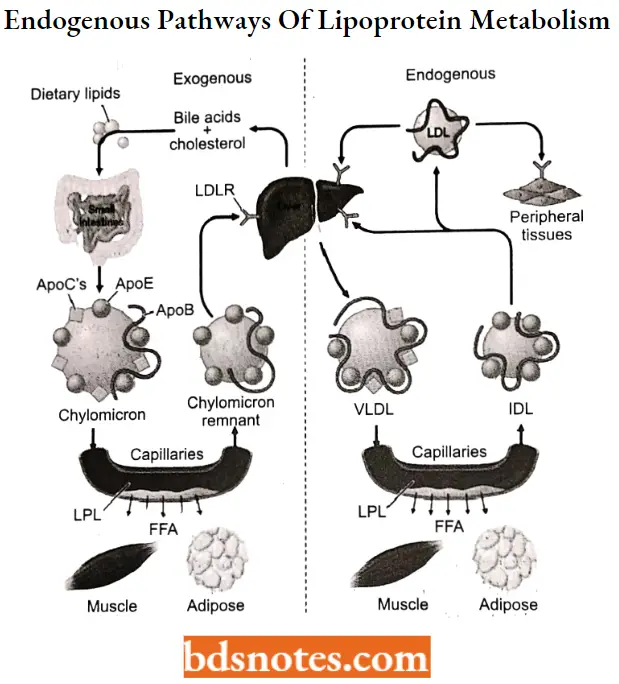
Lipids are transported by both exogenous (dietary intake) and endogenous (synthetic) pathways. The exogenous pathway begins after the ingestion of a fat-containing meal or snack.
- Dietary lipids are absorbed in the form of cholesterol and fatty acids. The fatty acids are then reesterified within the intestinal mucosal cells and, along with the cholesterol, are incorporated into chylomicrons, the largest lipoprotein.
- During circulation, chylomicrons are degraded into remnants by the action of lipoprotein lipase, a plasma membrane enzyme located on capillary endothelial cells in adipose and muscle tissue.
- The interaction of chylomicrons with lipoprotein lipase requires apolipoprotein (apo) C-II, and the absence of either the enzyme or the apolipoprotein can lead to hypertriglyceridemia and pancreatitis.
- The liberated free acids are then available for either storage or energy generation by these tissues. The remnants are predominantly cleared from the plasma by liver parenchymal cells via recognition of the apo E portion Of the carrier.
- In the endogenous pathway of lipid transport, lipids are secreted from the liver. These are triglycerides and cholesterol combined with apoprotein B-100 and apoprotein E to form VLDL.
The VLDL is acted on by lipoprotein lipase in the capillaries of adipose tissue to generate free fatty acids (FFAs) and an IDL. Some IDL binds to LDL receptors in the liver and is cleared from plasma by endocytosis.
- Approximately half of the circulating IDL is converted to LDL in the plasma by additional loss of triglycerides. This LDL has a half-life in plasma of about 1.5 days and represents 60% to 70% of the cholesterol in plasma.
- These LDL particles bind to LDL receptors in extrahepatic tissues and are removed from the plasma. Levels of LDL receptors vary depending on the need of extrahepatic tissues to bind- LDL to use cholesterol.
- The extrahepatic tissue subsequently releases HDL. Free plasma cholesterol can be adsorbed onto HDL and the cholesterol esters formed by the enzyme lecithin-cholesterol acyltransferase (LCAT).
- These esters are transferred from HDL to VLDL or LDL in plasma to complete the cycle.
Drug Therapy Affecting Lipoprotein Metabolism
In general, successful use of these compounds depends on proper identification and classification of the hyperlipoproteinemia affecting the patient.
HMG- CoA reductase inhibitors (Statins) For Example. Lovastatin, Atorvastatin.
Lipoprotein lipase activators (Fibrates) For Example. Clofibrate.
Bile acid sequestrants (resins) For Example. Cholestyramine, Colestipol.
Lipolysis and triglyceride synthesis inhibitors For Example. Nicotinic acid.
Cholesterol absorption inhibitor For Example. Ezetimibe
HMG-CoA Reductase Inhibitors (Statins)(HMGRIs)
- The development and use of HMGRIs began in 1976 with the discovery of mevastatin. Several years later, a structurally similar compound was isolated from Monascus ruber and Aspergillus terreus.
- This compound was originally known as mevinolin, was later renamed lovastatin, and was more than twofold more potent than mevastatin.
Statins SAR: Mevastatin and lovastatin served as lead compounds in the development of additional HMGRIs.
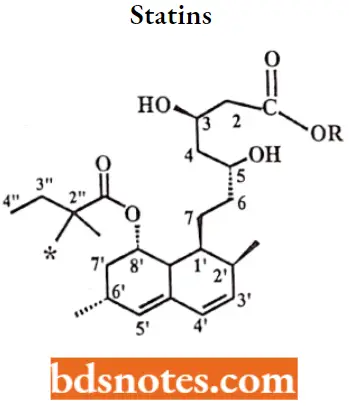
- The activity of HMGRIs is sensitive to the stereochemistry of the lactone ring, the ability of the lactone ring to be hydrolyzed, and the length of the bridge connecting the two ring systems.
- The bicyclic ring could be replaced with other lipophilic rings and the size and shape of these other ring systems were important to the overall activity of the compounds.
- Pravastatin, a ring-opened dihydroxy acid with a 6’a-hydroxyl group, is much more hydrophilic than either lovastatin or simvastatin.
- Proposed advantages of this enhanced hydrophilicity are minimal penetration into the lipophilic membranes of peripheral cells better selectivity for hepatic tissues, and a reduction in the incidence of side effects seen with lovastatin and simvastatin.
- The replacement of the bicyclic ring with various substituted, aromatic ring systems led to the development of atorvastatin (substituted pyrrole), fluvastatin (indole), pitavastatin (quinolone), and rosuvastatin (pyrimidine).
- Methyl substitution at the R2″ position increases activity (i.e., simvastatin is more potent than lovastatin).
Statins MOA: Inhibitors of HMG-CoA reductase lower plasma cholesterol levels by three related mechanisms: inhibition of cholesterol biosynthesis, enhancement of receptor-mediated LDL uptake, and reduction of VLDL precursors.
- As previously discussed, HMG-CoA reductase is the rate-limiting step in cholesterol biosynthesis. Inhibition of this enzyme causes an initial decrease in hepatic cholesterol.
- Compensatory mechanisms result in an enhanced expression of both HMG-CoA reductase and LDL receptors.
- The net result of all these effects is a slight to modest decrease in cholesterol synthesis, a significant increase in receptor-mediated LDL uptake, and an overall lowering of plasma LDL levels.
Lovastatin
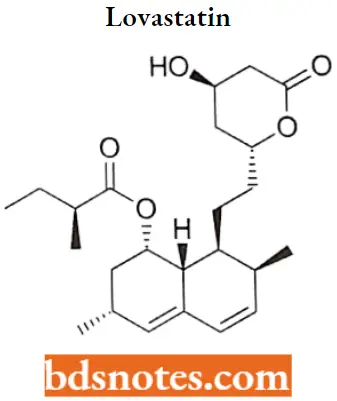
Lovastatin IUPAC name: 1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy6oxo-2H-pyran-2- yl)ethyl]-l-naphthalenyl ester.
Lovastatin Metabolism: Lovastatin is hepatically metabolized by CYP3A4 isozyme to the major active metabolites, the (3- hydroxy acid of lovastatin, the 6′-hydroxy derivative.
Lovastatin Uses: For management as an adjunct to diet to reduce elevated total -C, LDL-C, apo B, and TG levels in patients with primary hypercholesterolemia and mixed dyslipidemia.
For primary prevention of coronary heart disease and to slow the progression of coronary atherosclerosis in patients with coronary heart disease.
Lovastatin Adverse Effects: Gastrointestinal disturbances are the most common complaint; however, these and other adverse reactions tend to be mild and transient.
- Approximately 5% to 10% of patients will experience a mild increase in creatine phosphokinase levels; however, less than 1% will develop symptoms of myalgia and myopathy (For Example., fever, muscle aches or cramps, and unusual tiredness or weakness).
- Tests for creatine phosphokinase levels should be performed in patient muscle complaints.
Lipoprotein lipase activators (Fibrates)
A random screening test on a series of aryloxyisobutyric acids demonstrated that these compounds could lower both plasma cholesterol and total lipid levels.
- The compound that produced the best balance between activity and toxicity was ethyl p-chlorophenoxyisobutyrate.
- Later renamed clofibrate, this compound was subsequently shown to be a prodrug for p-chlorophenoxyisobutyric acid (clofibric acid).
Fibrates SAR:
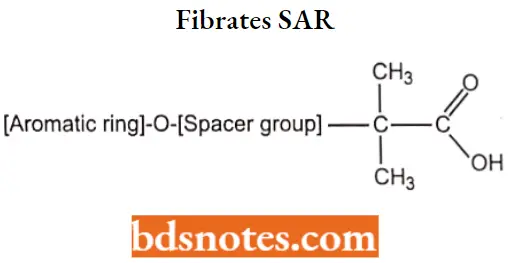
- The isobutyric acid group is essential for activity.
- Compounds containing an ester such as Fenofibrate, are required for vivo hydrolysis.
- Substitution at the para position of the aromatic ring with a chloro group or a chlorine-containing cyclopropyl ring produces compounds with significantly longer half-lives.
- Compounds contain a phenoxyisobutyric acid, the addition of an n-propyl spacer, as seen in gemfibrozil, results in an active drug.
Fibrates MOA: The mechanism of action has been proposed to be mediated through the activation of peroxisome proliferator-activated receptors (PPARs) and an alteration of gene expression.
- Specifically, fibrates bind to PPARa Decreases in plasma VLDL primarily result from the ability of these compounds to stimulate the activity of lipoprotein lipase, the enzyme responsible for removing triglycerides from plasma VLDL.
- Additionally, fibrates can lower VLDL levels through PPARa-mediated stimulation of fatty acid oxidation, inhibition of triglyceride synthesis, and reduced expression of apo C-3.
- This latter effect enhances the action of lipoprotein lipase because apo C-3 normally serves as an inhibitor of this enzyme.
- Favorable effects on HDL levels appear to be related to increased transcription of apoA-I and apo A-2 as well as a decreased activity of cholesteryl ester transfer protein.
Clofibrate
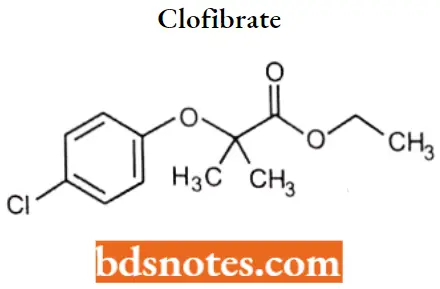
Clofibrate IUPAC name: ethyl 2-(p-chlorophenoxy)2-methylpropionate.
Clofibrate Metabolism: It undergoes rapid de-esterification in the gastrointestinal tract and or on first-pass metabolism to produce the active form, clofibric acid (chlorophenoxy isobutyric acid [CPIB]).
Clofibrate Uses: For Primary Dysbetalipoproteinemia (Type III hyperlipidemia) that does not respond adequately to diet. This helps control high cholesterol and high triglyceride levels.
Clofibrate Adverse effect: Clofibrate is tolerated well by most patients; the most common side effects are nausea and, to a lesser extent, other gastrointestinal distress.
The dosage of anticoagulants, if used in conjunction with this drug, should be reduced by one-third to one-half, depending on the individual response, so that the prothrombin time may be kept within the desired limits.
Bile acid sequestrants (resins)
Cholestyramine, colestipol, and colesevelam are chemically classified as anion-exchange resins. This term arises from their ability to selectively bind and exchange negatively charged atoms or molecules with one another.
- The selectivity comes from the fact that these positively charged resins do not bind equally to all anions.
- For example, the chloride ion of cholestyramine can be displaced by, or exchanged with, other anions (For example., bile acids) that have a greater affinity for the positively charged functional groups on the resin.
Resins SAR:
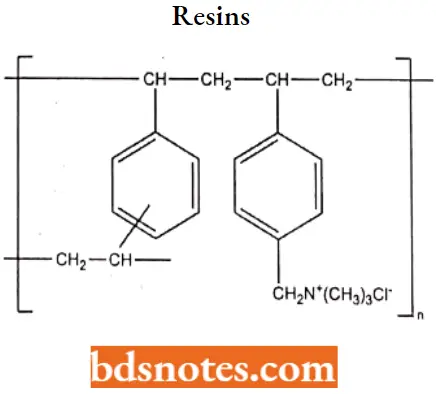
- Cholestyramine is a copolymer consisting primarily of polystyrene, with a small amount of divinylbenzene as the cross-linking agent.
- It contains quaternary ammonium groups that function as binding sites for anions.
- Increasing the amount of divinylbenzene from 2% to 4% to 8% increases the cross-linkage and reduces the porosity of the resin. This prevents the binding of bile acids and decreases the efficacy of the compound.
- Colestipol is a copolymer of tetraethylenepentamine and epichlorohydrin contains basic secondary and tertiary amines.
- The total nitrogen content of colestipol is greater than that of cholestyramine, the functional anion-exchange capacity of the resin depends on intestinal pH and may be less than cholestyramine.
- Cholestyramine has a higher adsorption capacity than colestipol for bile salts.
- Colesevelam is a more diverse polymer. In the case of colesevelam, poly (allylamine) is initially cross-linked with epichlorohydrin and then alkylated with 1-bromodecane and (6-bromohexyl)- trimethylammonium bromide.
Resins MOA: They bind the two major bile acids, glycocholic acid, and taurocholic acid, and greatly increase their fecal excretion. As a result, decreased concentrations of these compounds are returned to the liver.
- This removes the feedback inhibition of 7a-hydroxylase and increases the hepatic conversion of cholesterol to bile acids.
- The decrease in hepatic cholesterol concentrations leads to several compensatory effects: increased expression of LDL receptors, increased hepatic uptake of plasma LDL, induction of HMG-CoA reductase, and increased biosynthesis of cholesterol.
- The latter two effects are insufficient to counteract the increases in cholesterol clearance and catabolism; however, concurrent use of an HMGRI can provide an additive effect in lowering LDL cholesterol.
- The decreased return of bile acids to the liver also will produce an increase in triglyceride synthesis and a transient rise in VLDL levels.
- Subsequent compensatory mechanisms will increase VLDL removal, most likely through the increased LDL receptors, and return VLDL levels to predrug levels.
Cholestyramine
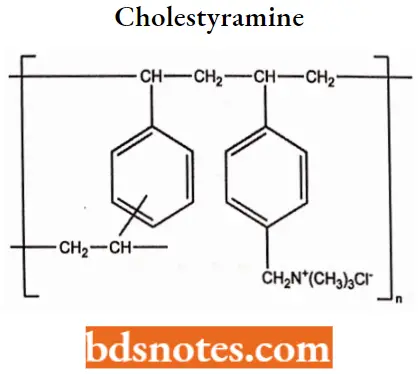
Cholestyramine MOA: Cholestyramine binds bile in the gastrointestinal tract to prevent its reabsorption.
- The resin is a strong anion exchange resin, which means that it can exchange its chloride anions with anionic bile acids in the gastrointestinal tract and bind them strongly in the resin matrix.
- The functional group of the anion exchange resin is a quaternary ammonium group attached to an inert styrene-divinylbenzene copolymer.
Cholestyramine Uses: Indicated as adjunctive therapy to diet for the reduction of elevated serum cholesterol in patients with primary hypercholesterolemia (elevated low-density lipoprotein [LDL] cholesterol) who do not respond adequately to diet. Also for the relief of pruritus associated with partial biliary obstruction.
Cholestyramine Adverse Effect: Constipation is by far the most frequent patient complaint. Increasing dietary fiber or using bulk-producing laxatives, such as psyllium, often can minimize this adverse effect.
- Other gastrointestinal symptoms, such as bloating and abdominal discomfort, usually disappear with continued use; however, the possibility of fecal impaction requires that extreme caution be used in patients with preexisting constipation.
- All three of these compounds release chloride ions as part of their exchange mechanism and can cause hyperchloremic acidosis.
- This is not a common occurrence, but it may limit the use of bile acid sequestrants in patients with renal disease. Hypoprothrombinemia and bleeding are caused by the ability of bile acid sequestrants to bind with and impair the absorption of dietary vitamin K.
- These effects also are rare, but they may limit the use of these agents in patients with preexisting clotting disorder and those being concurrently treated with anticoagulants.
Colestipol
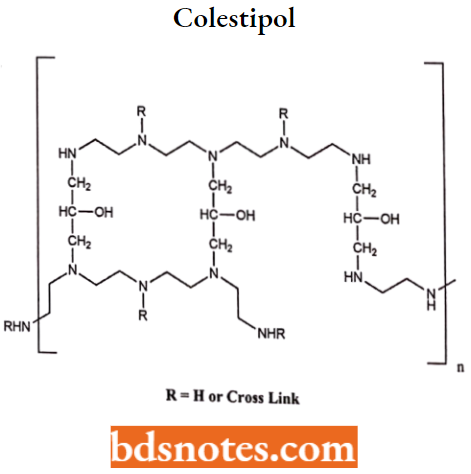
Colestipol MOA: It functions as an anion-exchange, resin-requesting gent like that of cholestyramine resin.
Colestipol hydrochloride reduces cholesterol levels without affecting triglycerides and seems to be especially effective in the treatment of type 2 hyperlipoproteinemias.
Lipolysis and triglyceride synthesis inhibitors
Nicotinic Acid
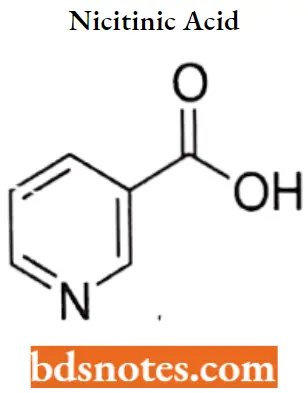
Nicotinic Acid IUPAC name: pyridine-3-carboxylic acid.
Nicotinic Acid MOA: The mechanism by which niacin exerts its lipid-lowering effects is not entirely understood, but may involve several actions, including a decrease in the esterification of hepatic triglycerides.
Niacin treatment also decreases the serum levels of apolipoprotein B-100 (apo B), the major protein component of the VLDL (very low-density lipoprotein) and LDL fractions.
Nicotinic Acid Metabolism: Nicotinic acid is a B-complex vitamin that is converted to nicotinamide, nicotinamide adenine dinucleotide (NAD+), and NADP+.
- The latter two compounds are coenzymes and are required for oxidation or reduction reactions in a variety of biochemical pathways.
- Additionally, nicotinic acid is metabolized into several inactive compounds, including nicotinic acid and N-methylated derivatives.
Nicotinic Acid Uses: Nicotinic acid is approved for the treatment of hypercholesterolemia, hypertriglyceridemia, and familial combined hyperlipidemia in patients who have not responded to diet, exercise, and other non-pharmacologic methods.
- It also is approved for nutritional supplementation, for the prevention of pellagra, and as an adjunct therapy for peripheral vascular disease and circulatory disorders.
- It is contraindicated in patients with hepatic disease and peptic ulcer disease. Additionally, because of its ability to elevate glucose and uric acid levels.
- Especially when taken in large doses, nicotinic acid should be used with caution in patients who have or are predisposed to diabetes mellitus and gout.
Nicotinic Acid Adverse effects: The most common (and, often, dose-limiting) side effects of nicotinic acid treatment are cutaneous vasodilation (flushing and pruritus) and gastrointestinal intolerance, which may occur in 20% to 50% of treated patients.
- Flushing and pruritus are prostaglandin-mediated effects and may be prevented by taking aspirin or indomethacin before nicotinic acid.
- Gastrointestinal side effects, such as flatulence, nausea, vomiting, and diarrhea, can be minimized if nicotinic acid is taken either with or immediately after meals.
Cholesterol Absorption Inhibitor
Ezetimib
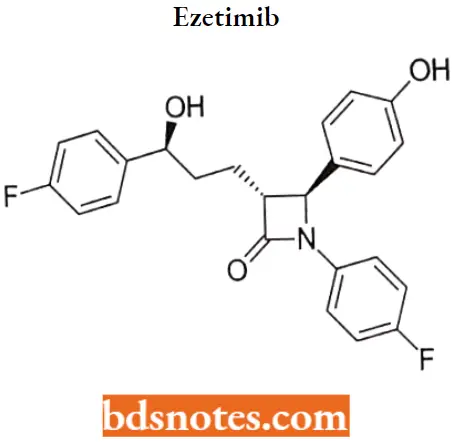
Ezetimib IUPAC name: (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one.
Ezetimib MOA: Ezetimibe lowers plasma cholesterol levels by inhibiting the absorption of cholesterol at the brush border of the small intestine.
- Specifically, ezetimibe has been proposed to bind to a specific transport protein located in the wall of the small intestine, resulting in a reduction of cholesterol transport and absorption.
- Ezetimibe appears to be selective in its actions in that it does not interfere with the absorption of triglycerides, lipid-soluble vitamins, or other nutrients.
- The decreased absorption of cholesterol eventually leads to enhanced receptor-mediated LDL uptake similar to that seen with bile acid sequestrants and HMGRIs.
Ezetimib Metabolism: ezetimibe is rapidly and extensively metabolized in the intestinal wall and the liver to its active metabolite, a corresponding phenol glucuronide.
This glucuronide is excreted in the bile back to its active site. A small amount (<5%) of ezetimibe undergoes oxidation to convert the benzylic hydroxyl group to a ketone; however, ezetimibe does not appear to exert any significant effect on the activity of CYP450 enzymes.
Ezetimib Adverse Effect: Abdominal pain, diarrhea, arthralgia, back pain, cough, pharyngitis, sinusitis, fatigue, and viral infection.
Antihyperlipidemic Agents Multiple Choice Questions And Answers
Question 1. Which of the following is true concerning lipoproteins?
- They serve to transport lipids from tissue to tissue and participate in lipid metabolism.
- The inside of the particles has varying amounts of neutral lipids, cholesterol esters, and triacylglycerols.
- 5 major classes have specific physiological and anatomical significance. The classes are chylomicrons, VLDL, IDL, LDL, and HDL.
- All of the above are true concerning lipoproteins.
Answer: 4. All of the above are true concerning lipoproteins.
Question 2. Which apolipoprotein components of chylomicrons stimulate lipase?
- C2 and E
- A1 and A2
- B-48 and E
- C3 and A4
Answer: 1. C2 and E
Question 3. Which drug to treat hyperlipidemia acts by inhibiting the mobilization of free fatty acids (FFA) from peripheral adipose tissue to the liver?
- Statins
- Fabric Acids
- Niacin (Nicotinic Acid)
- Bile acid-binding Resins
Answer: 3. Niacin (Nicotinic Acid)
Question 4. Which antihyperlipidemic is most effective when given in combination with other drugs?
- Niacin
- Bile acid-binding resins
- Ezetimibe
- Statins
Answer: 1. Niacin
Question 5. What is the drug of choice for lowering VLDL levels in patients at risk of pancreatitis and for mixed elevations of LDLs and VLDLs and low levels of HDLs?
- Fabric Acids
- Ezetimibe
- Statins
- Niacin
Answer: 4. Niacin
Question 6. How can you avoid some of the adverse effects seen with Niacin (dyspepsia, skin flushing, itching)?
- By taking an NSAID like Aspirin concurrently.
- By taking the medication at night.
- By taking the medication with food.
- By taking the medication on an empty stomach.
Answer: 1. By taking an NSAID like Aspirin concurrently.
Question 7. What do the fibrous acid derivatives do?
- Enhance oxidation of FA in the liver and muscle.
- Reduce the rate of lipogenesis in the liver.
- Increase synthesis of Apo AI and II for more HDL.
- All of the above.
Answer: 4. All of the above.
Question 8. How do the Bile Acid-Binding Resins (Cholestyramine, Colestipol, and Colesevelam) function?
- By binding LDLs and removing them from circulation.
- By anionic exchange.
- By attacking the plaque directly.
- By cationic exchange.
Answer: 4. By cationic exchange.
Antihyperlipidemic Agents Short Questions And Answers
Question 1. What are lipoproteins? Classify them.
Answer:
Lipoproteins are macromolecules consisting of lipid substances (cholesterol, triglycerides) noncovalently bound with protein and carbohydrates.
The various lipoproteins found in plasma are chylomicrons, very-low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL).
Question 2. Write MOA of HMG CoA reductase inhibitor.
Answer:
Inhibitors of HMG-CoA reductase lower plasma cholesterol levels by three related mechanisms: inhibition of cholesterol biosynthesis, enhancement of receptor-mediated LDL uptake, and reduction of VLDL precursors.
- As previously discussed, HMG-CoA reductase is the rate-limiting step in cholesterol biosynthesis. Inhibition of this enzyme causes an initial decrease in hepatic cholesterol.
- Compensatory mechanisms result in an enhanced expression of both HMG-CoA reductase and LDL receptors.
- The net result of all these effects is a slight to modest decrease in cholesterol synthesis, a significant increase in receptor-mediated LDL uptake, and an overall lowering of plasma LDL levels.
Question 3. Discuss the S AU of Flbrates.
Answer:
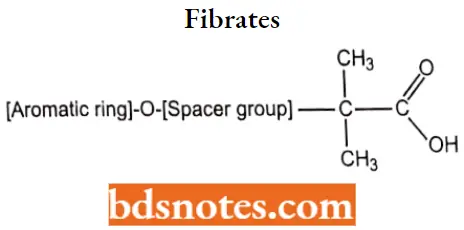
- The isobutyric acid group is essential for activity.
- Compounds containing an ester such as Fenofibrate, are required for vivo hydrolysis.
- Substitution at the para position of the aromatic ring with a chloro group or a chlorine-containing.
- Cyclopropyl ring produces compounds with significantly longer half-lives.
- Compounds contain a phenoxyisobutyric acid, the addition of an n-propyl spacer, as seen in gemfibrozil, results in an active drug.
Question 4. Give clinical uses of Cholestyramine.
Answer:
Indicated as adjunctive therapy to diet for the reduction of elevated serum cholesterol in patients with primary hypercholesterolemia (elevated low-density lipoprotein [LDL] cholesterol) who do not respond adequately to diet. Also for the relief of pruritus associated with partial biliary obstruction.

Leave a Reply