Antidiabetic Agents Introduction
The pancreas secretes digestive enzymes, glucagon, and insulin. An isolated group of cells within a cell’s pancreas is called an islet of Langerhans. These cells are divided into three types (secretes glucagon), β cells (secretes insulin), and γ cells (secretes somatostatin).
Insulin plays an important role in the digestion and utilization of food substances. It is essential for the phosphorylation of glucose to glucose-6-phosphate. Glucose-6-phosphate is further catabolized to give energy.
In some individuals glucose levels in blood increase due to lack of sufficient insulin. This condition is called hyperglycemia. The disease is known as diabetes mellitus.
Some of the more important- symptoms associated with the disease are polydipsia, polyurea, ketonemia, and ketonuria. Most patients can be classified clinically as:
- Insulin-dependent diabetes mellitus (Type-1 diabetes)-Type-1 diabetes is an auto-immune disease caused by the destruction of pancreatic islet cells.
- Non-insulin-dependent diabetes mellitus (Type-2 diabetes). – Type-2 diabetes the cause of hyperglycemia is a combination of insulin resistance and a loss of secretory function by the pancreatic β-cells.
Read and Learn More Medicinal Chemistry II Notes
Hypoglycemic Agents
Hypoglycemic agents or antihyperglycemic or antidiabetic agents lower the blood sugar and are used to treat the symptoms of diabetes mellitus.
- Pharmacologic treatment for type 1 diabetics requires intensive insulin therapy. The large number of short- and long-acting insulin analogs allows for the use of multiple doses of basal and prandial (at meals) doses of insulin.
- Therefore, patients can match their dose of prandial insulin to carbohydrate intake, pre-meal plasma glucose, and anticipated activity.
The most common side effect of the use of insulin is severe hypoglycemia; however, the development of quick-acting and long-acting insulin analogs has moderated this side effect while still maintaining equal HbAic (Hemoglobin A) lowering.
- Medical treatment of the type 2 diabetic requires management of hyperglycemia as measured by the patient’s HbA1c below 7%.
- Obesity and a sedentary lifestyle are the major risk factors for diabetes; therefore, weight Ions, dietary changes, and Increased activity levels should be the initial approach to treating type 2 diabetes. In addition to Insulin, there are several classes of oral hypoglycemic agents available.
Antidiabetic Agents Classification
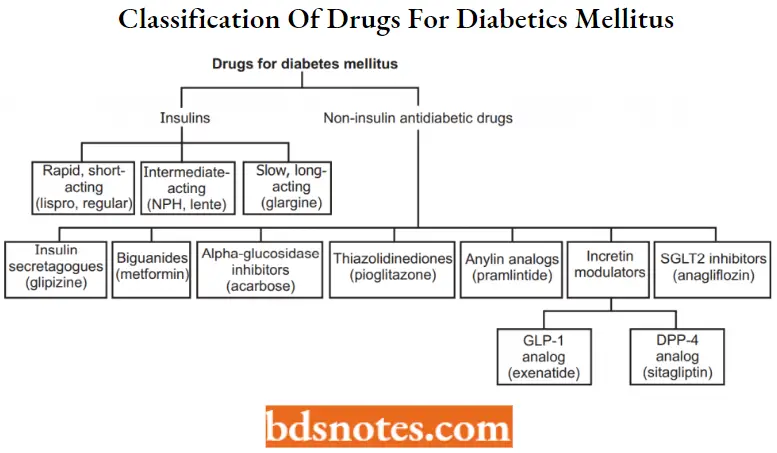
Insulin and its Preparations
Structure of insulin
The insulin molecule is composed of two polypeptide chains (A and B) linked together by two disulfide bonds. There is an additional disulfide bond in chain A.
- Chain A contains 21 amino acid residues, and chain B has 30 amino acids, giving a molecular weight of 5,734 Daltons. Insulin is biosynthesized in the b-cells of the pancreas from preproinsulin, a 110 amino acid chain with a molecular weight of 12,000 Daltons.
- Preproinsulin is cleaved in the endoplasmic reticulum, losing a 24- -amino acid unit from the N-terminus. The product is called proinsulin (molecular weight =*ÿ 9,000 Daltons), which folds to allow the disulfide bonds to form.
- And undergoes further proteolytic modification in the Golgi apparatus, losing four basic amino acids (ArgB31, ArgB32, LysA64, and ArgA65) and releasing connector C-chain by the action of prohormone convertases PCI and PC2.
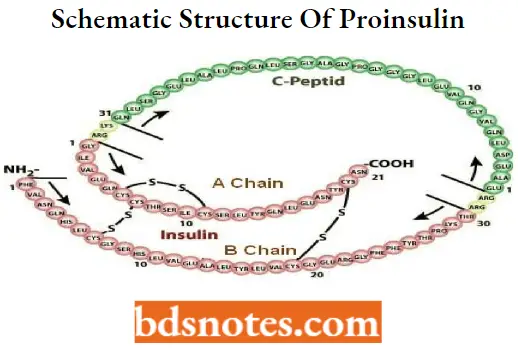
The biologically active form of insulin is the monomer. However, in solution, insulin can exist as a dimer and as a hexamer (six monomeric units attached).
The hexamer is formed by its coordination with two zinc ions, the storage form of insulin in the granules of the b-cells. When released from the granules, the hexamer gets diluted in the plasma (nanomolar) and dissociates into monomers.
Sources and stability of insulin
- Historically, patients only had the option of administering either bovine-based or porcine-based insulin, which were alternatives to human insulin because their amino acid sequence homology between species was superb.
- However, animal sources have become less relevant and have fallen into disuse. Today, the following sources of insulin are available: biosynthetic human, semisynthetic human, and analogs of human insulin. Human insulin is the least antigenic of the available insulins and tends to be more soluble than animal insulin.
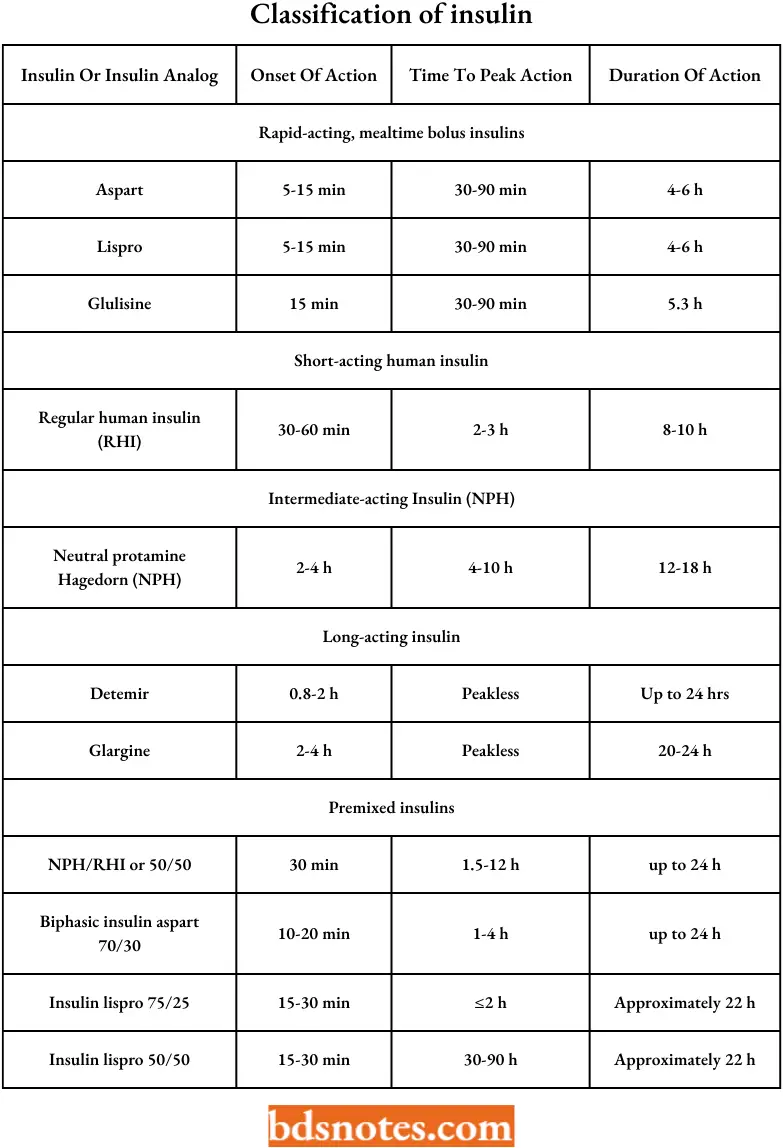
Types of Insulin
The insulin analogs available for the treatment of diabetes are classified according to their rate of onset and duration of action.
Structure-activity relationship studies revealed that variations or removal of amino add residues from the C-terminus of the B chain could influence the rate of dimer formation while not drastically changing the biological activity.
- Rapid-acting insulin analogs include insulin lispro, insulin aspart, and insulin glulisine. All have changes made to the amino add residues in the C-terminus of the B chain.
- Insulin lispro– the LysB29 is switched with ProB28.
- Insulin aspart– the ProB28 has been substituted with an Asp.
- Insulin Glulisinc-ValB3 is substituted with a Lys, and LysB29 is changed to glutamate (Glu).
- Short-acting insulin– Regular human insulin is the prototype. Making regular insulin a good choice for intravenous treatment of diabetics
- Intermediate-acting insulin-Include Insulin NPH and insulin lente.
- Insulin NPH is prepared by adding stoichiometric (equal) amounts of the positively charged
polypeptide protamine to regular insulin. - Insulin lente is Prepared by combining regular insulin and zinc in an acetate buffer to form a crystalline complex that dissolves slowly in subcutaneous fluids.
- Insulin NPH is prepared by adding stoichiometric (equal) amounts of the positively charged
- Long-acting insulin)
- Insulin glargine analog results from the replacement of AsnA21 by glycine (Gly) and the addition of two Arg amino acids to the C-terminus of the B chain.
- Insulin ultralente– is a long-acting insulin that is a four-zinc acetate crystalline product.
- Insulin detemir is the newest long-acting analog. This analog results from the N-acylation of the LysB29 with the 14-carbon myristic acid.
- A new long-acting insulin analog currently in phase 3 clinical trials is insulin degludec. Insulin deglude results from the removal of GluB30 and N-acylation of LysB29 with L-g-Glu amidated with hexadecanoic acid.
- Premixed insulins– Different types of insulin can be premixed in the same syringe and are usually prescribed for patients needing a simple insulin treatment plan. NPH insulin can be premixed with either aspart or lispro rapid-acting insulins.
- Mixtures of NPH and lispro can be 50:50 or 75:25, whereas NPH and aspart are available in 70:30 ratios. A 70:30 NPH/regular insulin premix is also available.
- The benefit of using premixed insulin is that rapid-acting and long-acting insulin can be administered at the same time and can be given twice a day, usually at breakfast and supper.
- The drawback of using such a regimen is that to be effective, the amount of carbohydrates to be eaten at each meal is preset.
Classification of insulin preparations with their pharmacokinetics
Non-insulin antidiabetic or oral hypoglycemic agents
A characteristic of type 2 diabetes is diminished insulin secretion due to impaired b-cell function and or insulin resistance of peripheral tissues such as the liver, adipose, and skeletal muscle, which causes hyperglycemia. The current line of treatment includes:
- Insulin secretagogucs
- Insulin sensitizers
- Bigunnldes
- a-glucosidase inhibitors
- GLIM analogs and DPP-IV inhibitors
- Amylin agonists
- Insulin secretagogucs
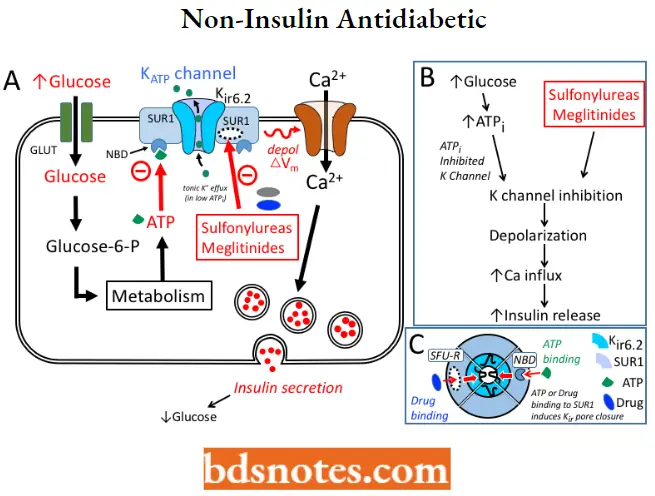
The insulin secretagogues include sulfonylureas and meglitinides, and both increase insulin release from the pancreas by a common mechanism.
Non-Insulin antidiabetic MOA: All the sulfonylureas and meglitinides stimulate the release of insulin from the B-cells of the pancreas. These cells metabolize glucose in the mitochondria to produce ATP, which increases the intracellular ratio of ATP/ADP, resulting in the closure of the ATP-sensitive K+ channel on the plasma membrane.
- Closure of this channel triggers the opening of voltage-sensitive Ca2+ channels, leading to a rapid influx of Ca2+.
- Increased intracellular Ca2+ causes an alteration in the cytoskeleton and stimulates the translocation of insulin-containing granules to the plasma membrane and the exocytotic release of insulin.
- The ATP-sensitive K+ channel is an octameric heterocomplex consisting of two units of the binding site for both sulfonylureas and ATP designated as the sulfonylurea receptor type 1 (SUR1) and an inwardly rectifying K+ channel.
Sulfonylurea binding to the SURl causes the same effect as an increase in the ATP/ADP ratio, which is to close the channel leading to the secretion of insulin.
Sulfonylureas
Sulfonylureas are classified as First generation For Example. Tolbutamide, Chlorpropamide.
Second generation For Example. Glipizide, Glimepiride.
Sulfonylureas SAR: The benzene ring should contain one substituent, preferably at the para position (R). The substituents that seem to enhance hypoglycemic activity are methyl, amino, acetyl, chloro, bromo, methylation, and trifluoromethyl groups.
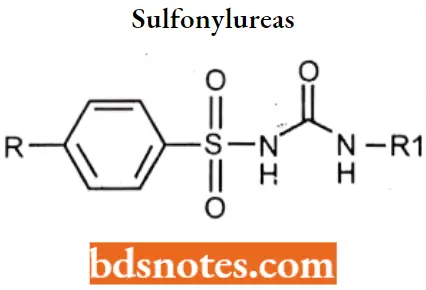
- Compounds with p-(-β-arylcarboxamidoethyl) substituents (the second-generation agents) have better activity than the first-generation agents. It is believed that this is because of a specific distance between the nitrogen atom of the substituent and the sulfonamide nitrogen atom.
- The group attached to the terminal nitrogen (R2) should be of a certain size and should impart lipophilic properties to the molecule. The N-methyl is inactive, N-ethyl has low activity, while N-propyl to N-hexyl is most active. Activity is lost if the N-substituent contains 12 or more carbons.
Sulfonylureas Adverse effects: Sulfonylureas are associated with weight gain, though less so than insulin. Due to their mechanism of action, sulfonylureas may cause hypoglycemia and require consistent food intake to decrease this risk. The risk of hypoglycemia is increased in elderly, debilitated, and malnourished individuals.
Tolbutamide
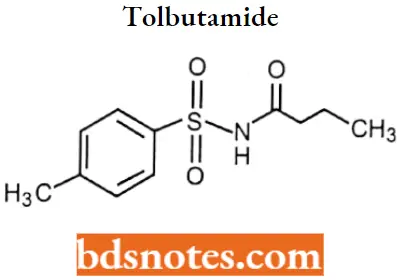
Tolbutamide IUPAC name: 3-butyl-1-(4-methylbenzenesulfonyl)urea.
Tolbutamide Metabolism: Metabolized in the liver principally via oxidation of the p-methyl group producing the carboxyl metabolite, 1-butyl-3-p-carboxyphenylsulfonylurea.
- May also be metabolized to 4- 4-hydroxy tolbutamide. Unchanged drugs and metabolites are eliminated in the urine and feces.
- Approximately 75-85% of a single orally administered dose is excreted in the urine principally as the l-butyl-3-p-carboxyphenylsulfonylurea within 24 hours.
Tolbutamide Uses: For treatment of NIDDM (non-insulin-dependent diabetes mellitus) in conjunction with diet and exercise.
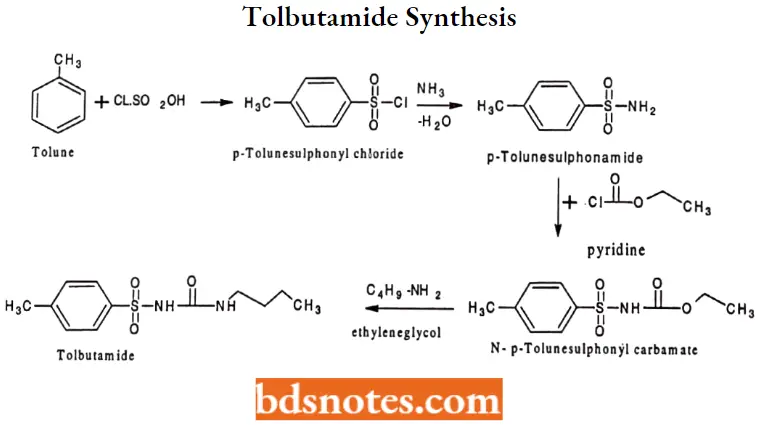
Tolbutamide Synthesis:
Chlorpropamide
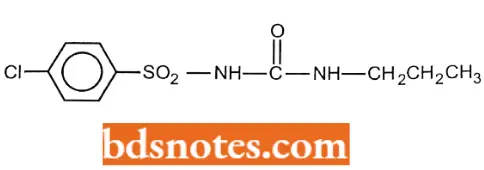
Chlorpropamide IUPAC name: 1-(4-chlorobenzenesulfonyl)-3-propylurea.
Chlorpropamide Metabolism: Chlorpropamide has a considerably longer half-life than the other sulfonylureas and, as a result, has a greater tendency for adverse effects. One explanation for the long half-life is that its metabolism (w and w-1 hydroxylation of the propyl group) is slow. A significant amount of the drug (~20%) is excreted unchanged.
Chlorpropamide Uses: For treatment ofNIDDMin conjunction with diet and exercise.
Glipizide
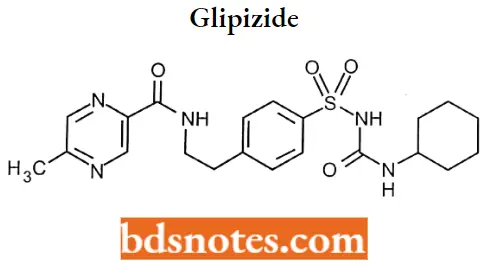
Glipizide IUPAC name: N-[2-(4-{[(cyclohexylcarbamoyl)amino]sulfonyl}phenyl)ethyl]-5-methylpyrazine-2-carboxamide.
Glipizide Metabolism: It undergoes hepatic metabolism. The major metabolites of glipizide are products of aromatic hydroxylation and have no hypoglycemic activity 3-cis-Hydroxyglipizide, 4-transHydroxyglipizide, and 4-trans-OH-glipizide.
A minor metabolite that accounts for less than 2% of a dose, an acetylamino ethyl benzene derivative is reported to have 1/10 to 1/3 as many hypoglycemic activities as the parent compound.
Glipizide Uses: For use as an adjunct to diet for the control of hyperglycemia and its associated symptomatology in patients with non-insulin-dependent diabetes mellitus (NIDDM; type II), formerly known as maturity-onset diabetes, after an adequate trial of dietary therapy has proved unsatisfactory.
Glimepiride
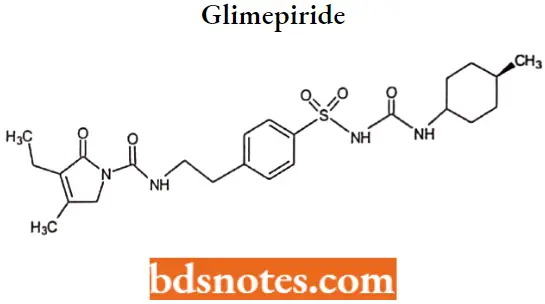
Glimepiride IUPAC name: 3-ethyl-2,5-dihydro-4-methyl-N-[2-[4[[[[(trans-4-methylcyclohexyl) amino] -carbonyl] amino]sulfonyl]phenyl]ethyl]-2-oxo-1H-pyrrole-l-carboxamide.
Glimepiride Metabolism: Glimepiride is metabolized in the liver, primarily by CYP2C9, to the active metabolite cyclohexyl hydroxymethyl derivative (M-l) which is then further metabolized to the inactive metabolite carboxyl derivativ (M-2).
Glimepiride Uses: For concomitant use with insulin for the treatment of noninsulin-dependent (type 2) diabetes mellitus.
Meglitinides: It is the benzoic acid derivative of the non-sulfonylurea moiety of glibenclamide.
Repaglinide
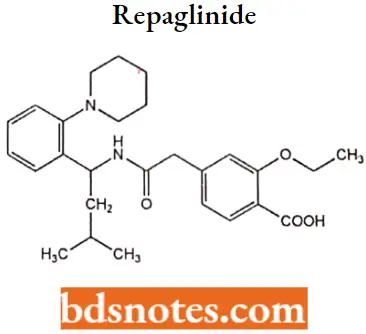
Repaglinide IUPAC name: 2-ethoxy-4-({[(lS)-3-methyl-1-[2-(piperidin-l-yl) phenyl] butyl] carbamoyl) methyDbenzoic acid.
Repaglinide Metabolism: Repaglinide is rapidly metabolized via oxidation and dealkylation by cytochrome P450 3A4 and 2C9 to form the major dicarboxylic acid derivative (M2). Further oxidation produces the aromatic amine derivative (Ml).
Glucuronidation of the carboxylic acid group of repaglinide yields an acyl glucuronide (M7). Several other unidentified metabolites have been detected. Repaglinide metabolites do not possess appreciable hypoglycemic activity.
Repaglinide Uses: As an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Repaglinide Adverse effects: Repaglinide has a rapid onset and short duration of action compared to other hypoglycemic drugs.
It is not associated with the prolonged hyperinsulinemia seen with the sulfonylureas, and possibly for this reason, it produces fewer side effects, including weight gain and potentially dangerous hypoglycemia.
Nateglinide
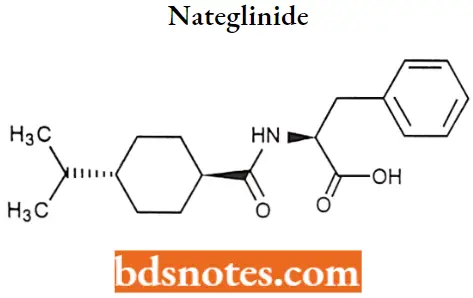
Nateglinide IUPAC name: (2R)-2-({hydroxy[(1r,4r)-4-(propan-2yl)cyclohexyl]methylidene}amino)-3-phenylpropanoic acid.
Nateglinide Metabolism: It is metabolized in the liver, with 16% excreted in the urine unchanged. The major metabolites are hydroxyl derivatives (CYP2C9, 70%; CYP3A4, 30%) that are further conjugated to glucuronide derivatives. The drug has an elimination half-life of 1.5 hours.
Nateglinide Uses: For the treatment of non-insulin dependent-diabetes mellitus in conjunction with diet and exercise.
Nateglinide Adverse Effects: May cause weight gain and hypoglycemia but lower than that of sulfonylurea.
Insulin sensitizers (peroxisome proliferatoiÿactivated receptor [PPAR] agonists)
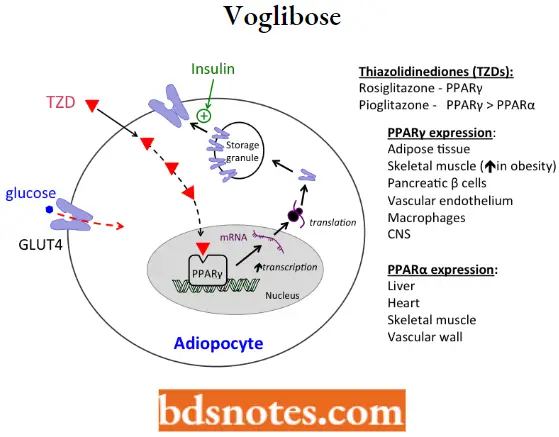
- The thiazolidinediones (TZDs) are classic examples of PPARg agonists and are commonly referred to as the “glitazones.” Thiazolidinediones (TZD) are ligands of the peroxisome proliferator-activated receptor-γ (PPAR-γ) that is expressed in the nucleus of adipocytes, myocytes, and hepatocytes.
- PPAR regulates the expression of genes involved in lipid and glucose metabolism, insulin signal transduction, and adipocyte differentiation. In diabetics, a major site of TZD action is adipose tissue.
Insulin sensitizers MOA: These drugs are synthetic ligands for the transcription factor PPARγ, a member of a superfamily of nuclear receptors including thyroid and steroid receptors. PPARγ is expressed in multiple tissue types (For Example. skeletal muscle, fat, and liver).
- Activation of PPAR-gamma receptors regulates the transcription of insulin-responsive genes involved in the control of glucose production, transport, and utilization. In this way, rosiglitazone enhances tissue sensitivity to insulin.
- As illustrated, one mechanism contributing to the hypoglycemic effect of thiazolidinediones is an increased expression of the glucose transporter GLUT4.
- The increased expression of GLUT4 (in addition to mediators of insulin signal transduction) increases the ability of cells (For Example. adipocytes) to take up glucose when stimulated by insulin.
Rosiglitazone
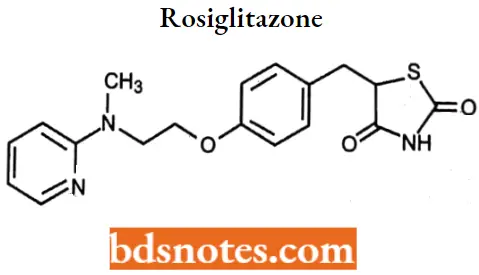
Rosiglitazone IUPAC name: 5-[4-[2-(N-methyl-N-(2-pyridyl) amino)ethoxy]benzyl]thiazolidine-2,4-dione.
Rosiglitazone Metabolism: It undergoes hepatic metabolism. Rosiglitazone is extensively metabolized in the liver to inactive metabolites via N-demethylation, hydroxylation, and conjugation with sulfate and glucuronic acid.
In vitro data have shown that Cytochrome (CYP) P450 isoenzyme 2C8 (CYP2C8) and to a minor extent CYP2C9 are involved in the hepatic metabolism of rosiglitazone.
Rosiglitazone Uses: Rosiglitazone is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Rosiglitazone Adverse effects: Side effects include fluid retention, congestive heart failure (CHF), and liver disease.
Pioglitazone
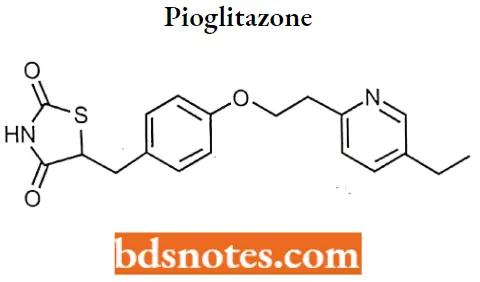
Pioglitazone IUPAC name: 5-({4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl}methyl)-l/3-thiazolidine-2,4-dione
Pioglitazone Metabolism: Pioglitazone is extensively metabolized by hydroxylation and oxidation; the metabolites also partly convert to glucuronide or sulfate conjugates. Metabolites M-3 and M-4 are the major circulating active metabolites in humans.
The cytochrome P450 isoforms involved are CYP2C8 and, to a lesser degree, CYP3A4 with additional contributions from a variety of other isoforms including the mainly extrahepatic CYP1A1.
Pioglitazone Uses: Pioglitazone is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Pioglitazone Adverse effects: Side effects include fluid retention, congestive heart failure (CHF), and liver disease.
Biguanides: The biguanides are chemically represented by the linkage of two guanidine groups with different side chains. The biguanides include metformin, phenformin, and buformin.
Metformin
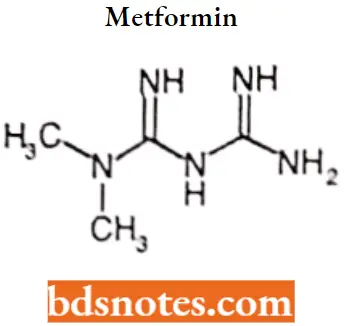
Metformin IUPAC name: 1-carbamimidamido-N, N-dimethylinethanimidamide
Metformin MOA: Although the mechanism of action is not completely understood, Metformin reduces hepatic glucose output by decreasing gluconeogenesis and stimulating glycolysis Current evidence suggests that results from a combination of intracellular effects in the liver.
- When metformin is taken orally, it is absorbed into hepatocytes from the portal vein through plasma membrane transporters, including the organic cation transporter 1 (OCT1).
- Inside the cell metformin inhibits mitochondrial respiratory-chain complex 1, resulting in reduced ATP levels and increased AMP.
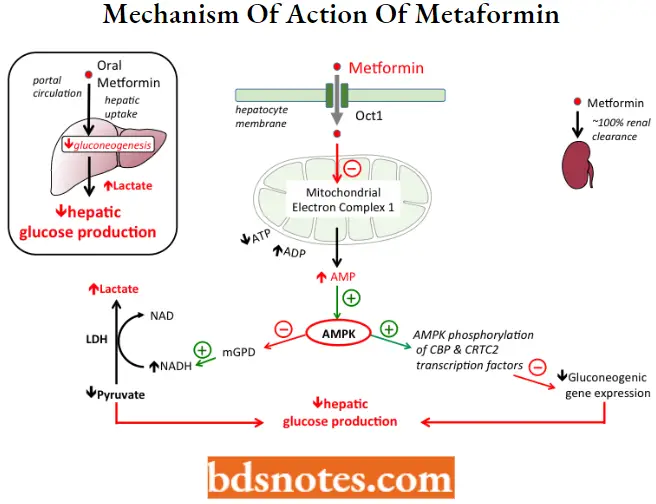
Increased AMP levels activate Adenosine Monophosphate-Activated Protein Kinase (AMPK), which contributes to the lowering of glucose production by at least 2 pathways:
- Increased AMPK phosphorylates CBP and CRTC2 transcription factors, which inhibits genes involved in the production of glucose (“gluconeogenic genes”);
- Increased AMPK also inhibits mitochondrial glycerol-3-phosphate dehydrogenase (mGPD), leading to an increase in cytosolic NADH, which both stimulates the conversion of pyruvate to lactate and simultaneously decreases gluconeogenesis.
- An accumulation of lactate to dangerous levels (lactic acidosis) can occur when metformin is taken by patients with other conditions resulting in metabolic acidosis
Metformin Metabolism: Metformin is excreted in the urine, via tubular excretion, as an un-metabolized drug with a half-life of approximately 2 to 5 hours; therefore, renal impairment and hepatic disease are contraindications for the drug.
Metformin Uses: For use as an adjunct to diet and exercise in adult patients (18 years and older) with non-insulin-dependent diabetes mellitus.
Metformin may also be used for the management of metabolic and reproductive abnormalities associated with polycystic ovary syndrome (PCOS). Metformin may be used concomitantly with a sulfonylurea or insulin to improve glycemic control in adults
Metformin Adverse effects: The most common adverse effects of metformin include: epigastric discomfort, nausea, flatulence, and vomiting. Diarrhea, drowsiness, weakness, dizziness, malaise, and headache may also occur.
- Metformin decreases liver uptake of lactate, thereby increasing lactate blood levels which may increase the risk of lactic acidosis
- In patients with decreased renal function, the plasma and blood half-life of metformin is
prolonged and the renal clearance is decreased.
α-glucosidase inhibitors
α-Amylase and α -α-glucosidase are key enzymes responsible for the metabolism of carbohydrates.
α-glucosidase inhibitors MOA: α-Glucosidase, which consists of maltase, sucrase, isomaltase, and glucoamylase, is a membrane-bound enzyme present in the brush border of the small intestine in relatively high concentrations in the proximal part of the jejunum.
- This enzyme catalyzes the conversion of the disaccharides sucrose and maltose into glucose.
- The resulting monosaccharides are then absorbed by the enterocytes of the jejunum and enter systemic circulation, as well as various biochemical pathways for the production of energy.
- Thus, inhibiting a-glucosidase will delay the process of carbohydrate absorption in the gut by moving these undigested disaccharides into the distal sections of the small intestine and colon. The result is the prevention of glucose production, thereby reducing postprandial hyperglycemia.
Acarbose
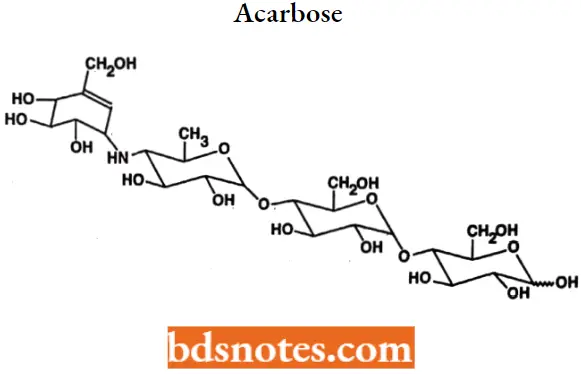
Acarbose IUPAC name: 0-4,6-dideoxy-4-[[(1S,4R,5S,6S)-4,5,6trihydroxy-3-(hydroxymethyl)-2-cyclohexen-1-yl]amino]a-D-glucopyranosyl-(1→4)-O- α -D-glucopyranosy-(1→4)D-glucose.
Acarbose Metabolism: Acarbose is only metabolized within the gastrointestinal tract by intestinal bacteria and also digestive enzymes to a lesser extent.
4-methyl pyrogallol derivatives (sulfate, methyl, and glucuronide conjugates) are the major metabolites. One metabolite (formed by cleavage of a glucose molecule from acarbose) also has α-glucosidase inhibitory activity.
Acarbose Uses: For treatment and management of diabetes type II (used in combination therapy as a second or third line agent).
Acarbose Adverse effects: Gastrointestinal symptoms are the most common reactions to acarbose.
Voglibose
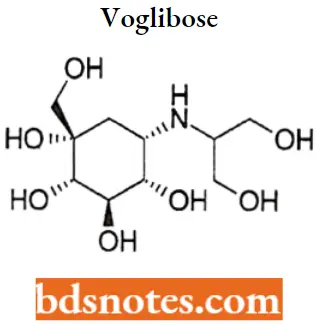
Voglibose IUPAC name: (1S,2S,3R,4S,5S)-5-[(1,3-dihydroxypropan-2-yl)amino]-1-(hydroxymethyl)cyclohexane-1,2,3,4-tetrol.
Voglibose Metabolism: Little metabolism occurs and no metabolites have as yet been identified. The half-life of voglibose is very similar to the one found for metformin and it is reported to be 4.08 hours.
Voglibose Uses: For the treatment of diabetes. It is specifically used for lowering post-prandial blood glucose levels thereby reducing the risk of macrovascular complications.
Its use can be extended to the treatment of glycosphingolipid lysosomal storage disease, HIV infections, and certain tumors.
Voglibose Adverse Effects: Gastrointestinal irritation, bloating, and flatulence caused by fermentation of undigested sugars in the large bowel by intestinal microflora.
GLP-1 analogs and DPP-4 inhibitors
GLP-1(Glucagon-like peptide) is a 36-amino acid peptide secreted by L-cells of the gut in response to a meal. It exerts control over glucose levels by promoting insulin secretion in a glucose-dependent manner.
- The role of GLP-1 was first proposed based on the observation that the amount of insulin secreted following an oral glucose dose exceeded that of an equivalent glucose dose administered intravenously in both diabetic and nondiabetic individuals.
- This observation was termed the incretin effect and is the result of two gut hormones, GLP-1 and GIP (glucose-dependent insulinotropic polypeptide.)
GLP-1 analogs and DPP-4 inhibitors MOA:
GLP-1 secretion from L-cells is similar to that of glucose-induced insulin secretion from pancreatic B-cells. Metabolism of glucose in the intestinal L-cells leads to the closure of ATP-linked potassium (K+) channels, resulting in depolarization of the membrane and entry of Ca2+, which leads to the secretion of GLP-1.
- GLP-1 is rapidly metabolized, with a half-life of 1 to 2 minutes, by an aminopeptidase enzyme, DPP-IV (dipeptidyl peptidase-IV), yielding an inactive peptide that is two amino acids shorter.
- It follows, therefore, that GLP-1 agonists or DDP-IV inhibitors would be effective agents to control blood glucose levels in diabetic patients.
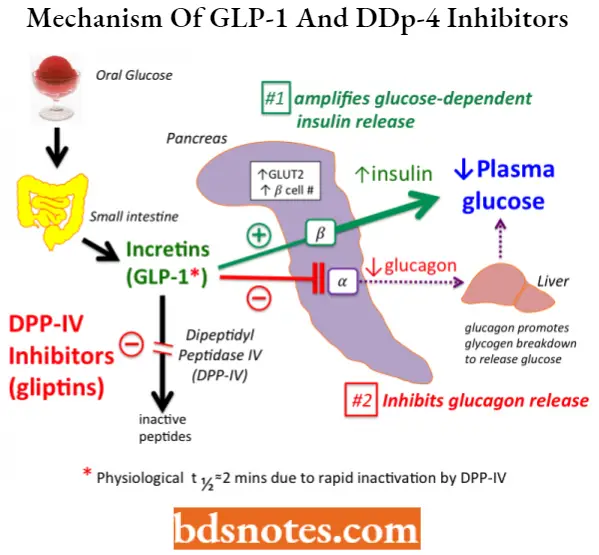
GLP-1 agonists– Exenatide, Liraglutide, Albiglutide (The major problem with GLP-1 analogs is their need for subcutaneous administration, which can limit patient compliance.)
DDP-IV inhibitors– Sitagliptin, Vildagliptin, Linagliptin, and Saxagliptin.
Amylin agonists: Amylin is a hormone that consists of a single chain of 37 amino acids and is released from pancreatic b-cells, co-secreted with insulin, and primarily involved in controlling postprandial glucose levels.
- Amylin, like insulin, shows similar fasting and postprandial patterns in healthy individuals by a variety of mechanisms, including delayed gastric emptying and suppression of glucagon secretion (not normalized by insulin alone).
- Which leads to a suppression of endogenous glucose output from the liver. Amylin also regulates food intake by modulating the appetite center of the brain.
The observation that amylin was deficient in both type 1 and type 2 diabetics stimulated research and development of amylin analogs that would be able to control postprandial glucose levels by:
- Modulation of gastric emptying.
- Prevention of postprandial rise in glucagon.
- Inhibition of caloric intake and potential weight gain.
Amylin itself is unsuitable as a drug because it aggregates and is insoluble in solution, which encouraged the development of chemical analogs.
Pramlintide is a chemical analog of amylin given enhanced water solubility and reduced aggregation liability by replacing the Ala25, Ser28, and Ser29 of the amylin peptide chain with prolines
Antidiabetic Agents Multiple Choice Questions And Answers
Question 1. What Oral Antidiabetic stimulates beta cells to secrete more insulin and increase receptor sites in the tissue?
- Alfa-Glucosidase Inhibitors
- Biguanides
- Sulfonylureas
- Thiazolidinediones
- Meglitinides
Answer: 3. Sulfonylureas
Question 2. What Oral Diabetic Medication has the following side effects: Diarrhea, Stomach upset, and Lactic acidosis?
- Miglitol
- Orinase
- Acarbose
- Metformin
- Tolinase
Answer: 4. Tolinase
Question 3. What Oral Medication should not be taken with Dairy products?
- Glyset
- Metformin
- Repaglinide
- Pioglitazone
- Glyburide
Answer: 2. Metformin
Question 4. What drug increases blood sugar by stimulating glycogenolysis (glycogen breakdown) in the liver?
- Glimepiride
- Glyburide
- Acarbose
- Miglitol
- Glucagon
Answer: 5. Glucagon
Question 5. What decreases the rate of liver glucose production, augments glucose uptake by tissues, and lowers lipids?
- Miglitol
- Nateglinide
- Pioglitazone
- Metformin
- Repaglinide
Answer: 4. Metformin
Question 6. What classification of OAD (Oral Anti-diabetic) medication delays the absorption of carbohydrates from the GI TRACT?
- Biguanides
- Thiazolidinediones
- Alpha- Glucosidase
- Meglitinides
- Sulfonylureas
Answer: 3. Meglitinides
Question 7. What increased glucose uptake in muscle, decreases glucose production in the liver?
- Meglitinides
- Thiazolidinediones
- Sulfonylureas
- Glucagon
- Biguanides
Answer: 2. Sulfonylureas
Question 8. What are the side effects of Meglitinides?
- Edema
- Weight gain
- Hypertension
- Diarrhea
- Weight loss
Answer: 2. Hypertension
Question 9. What are the Side effects of Thiazolidinediones?
- Weight loss
- Edema
- Hypoglycemia
- Hyperglycemia
- Diarrhea
Answer: 2. Hypoglycemia
Question 10. What stimulates the rapid and short-lived release of insulin from the pancreas
- Meglitinides
- Thiazolidinediones
- Biguanides
- Alpha- Glucosidase
- Sulfonylureas
Answer: 1. Meglitinides
Antidiabetic Agents Short Questions And Answers
Question 1. Give the types of insulin preparations.
Answer:
- Rapid-acting insulin analogs
- Insulin lispro
- Insulin Aspart
- Insulin Glulisine
- Short-acting insulin
- Regular insulin
- Intermediate-acting insulin
- Insulin NPH
- Insulin Lente
- Long-acting insulin
- Insulin glargine
- Insulin Ultralite
- Insulin detemir
- Insulin degludec
- Premixed insulins-D
Question 2. Classify insulin secretagogues with examples.
Answer:
Insulin secretagogues are classified as:
- Sulfonylureas
- First generation For Example. Tolbutamide, Chlorpropamide.
- Second generation For Example. Glipizide, Glimepiride.
- Meglitinides
- For Example. Repaglinide, Nateglinide.
Question 3. Write adverse effects of Metformin.
Answer:
The most common adverse effects of metformin include epigastric discomfort, nausea, flatulence, and vomiting. Diarrhea, drowsiness, weakness, dizziness, malaise, and headache may also occur.
- Metformin decreases liver uptake of lactate, thereby increasing lactate blood levels which may increase the risk of lactic acidosis.
- In patients with decreased renal function, the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased.
Question 4. Give the mode of action of GLP-1 analogs and DPP-4 inhibitors.
Answer:
GLP-1 secretion from L-cells is similar to that of glucose-induced insulin secretion from pancreatic B cells. Metabolism of glucose in the intestinal L-cells leads to the closure of ATP-linked potassium (K+) channels, resulting in depolarization of the membrane and entry of Ca2+, which leads to the secretion of GLP-1.
- GLP-1 is rapidly metabolized, with a half-life of 1 to 2 minutes, by an aminopeptidase enzyme, DPP-4 (dipeptidyl peptidase-4), yielding an inactive peptide that is two amino acids shorter.
- It follows, therefore, that GLP-1 agonists or DDP-4 inhibitors would be effective agents to control blood glucose levels in diabetic patients.

Leave a Reply