Diuretics Introduction
Diuretics are drugs, which increase the rate of urine flow. However, clinically useful diuretics also increase the excretion of Na+ and an accompanying anion (negatively charged ion) like Cl–.
- Since NaCl is the major determinant of extracellular fluid volume, diuretics reduce extracellular fluid volume (decrease in edema) by decreasing total body NaCl content.
- Although continued use of diuretics causes sustained net loss of Na+, the time course for this effect is limited by compensatory mechanisms including activation of the renin-angiotensin-aldosterone pathway and the sympathetic nervous system.
- When blood is filtered at the glomerulus, the fluid that enters the proximal tubule is the developing urine. As the tubular fluid passes down the tubule, solutes (Na+, K+, Cl–) are removed from the fluid and returned to the blood (reabsorption).
- Diuretics inhibit the reabsorption of Na+ ions, thereby reducing the quantity of water in body fluids. The diuretics drugs are mainly used to treat two medically important conditions, edema and hypertension.
Diuretics are also useful in treating several other conditions including increased cranial or intraocular pressure, diabetes insipidus, hypercalcemia, acute mountain sickness, primary hyperaldosteronism, and osteoporosis.
Read and Learn More Medicinal Chemistry II Notes
Diuretics Classification
- Carbonic anhydrase inhibitors
- For Example. Acetazolamide, Methazolamide, Dichlorphenamide.
- Thiazides
- For Example. Chlorothiazide, Hydrochlorothiazide, Hydroflumethiazide, Cyclothiazide.
- Loop diuretics
- For Example. Furosemide, Bumetanide, Ethacrynic acid.
- Potassium-sparing diuretics
- For Example. Spironolactone, Triamterene, Amiloride.
- Osmotic diuretics
- For Example. Mannitol.
“What is medicinal chemistry of diuretics? A detailed notes and Q&A guide”
Carbonic anhydrase inhibitors
Carbonic anhydrase (CA) inhibitors are derived from the sulphonamides. The sulfonamide group (—SO2NH2) is essential for its activity.
In 1937 observed that sulphanilamide not only had antibacterial activity but also produced systemic acidosis and an alkaline urine (HCO3-excretion).
The carbonic anhydrase inhibitors must have an unsubstituted sulphamoyl (—SO2NH2) group. Some potent CA inhibitors have an aromatic group (phenyl or heterocycle) attached to the sulphamoyl group.
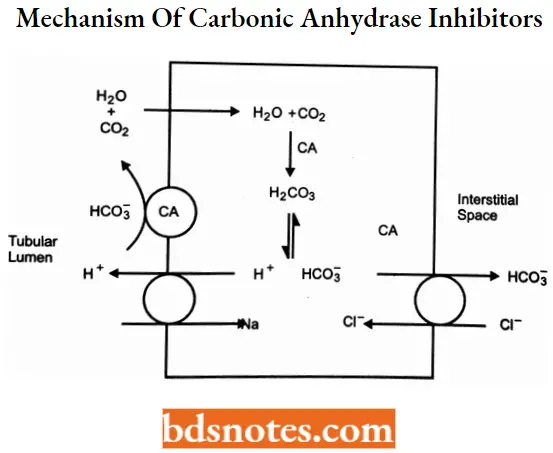
Carbonic anhydrase inhibitors MOA: This class of diuretics inhibits carbonic anhydrase enzymes in the membrane and cytoplasm of the epithelial cells. The primary site of action is the proximal tubules.
- These agents interfere with the reabsorption of HCO3 -. HCO3 – is reabsorbed in the proximal tubule and requires the activity of carbonic anhydrase.
- Intracellularly carbonic anhydrase converts H20 and CO2 to carbonic add (H2CO3). H2CO3 dissociates into H+ and HCO3-. The HCO3– is transported across the basolateral membrane.
- H+ is secreted into the tubular lumen in exchange for Na+. The H+ combines with a filtered HCO3– (using CA) to form H2CO3 which immediately dissociates into H2O and CO2 that, is reabsorbed.
- Therefore, filtered bicarbonate is reabsorbed for every H+ secreted. Carbonic anhydrase inhibitors, by blocking the enzyme, prevent the reabsorption of HCO3-.
- Accumulation of HCCV in the tubular lumen subsequently inhibits Na+ -H+ exchange and Na+ reabsorption.
The increase in sodium concentration in the tubular fluid may be compensated partially by increased NaCl reabsorption m later segments of the tubule. Thus, the diuretic effect of the carbonic anhydrase inhibitors is mild.
“Understanding diuretic agents through FAQs: Medicinal chemistry explained”
Acetazolamide
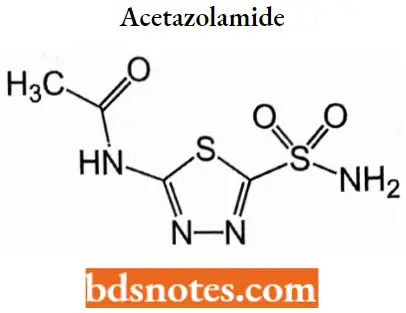
Acetazolamide IUPAC name: N-(5-sulfamoyl-l,3,4-thiadiazol-2-yl)acetamide.
Acetazolamide MOA: The diuretic effect depends on the inhibition of carbonic anhydrase, causing a reduction in the availability of hydrogen ions for active transport in the renal tubule lumen.
This leads to alkaline urine and an increase in the excretion of bicarbonate, sodium, potassium, and water.
Acetazolamide Uses: For adjunctive treatment of edema due to congestive heart failure; drug-induced edema; centrencephalic epilepsies; and chronic simple (open-angle) glaucoma.
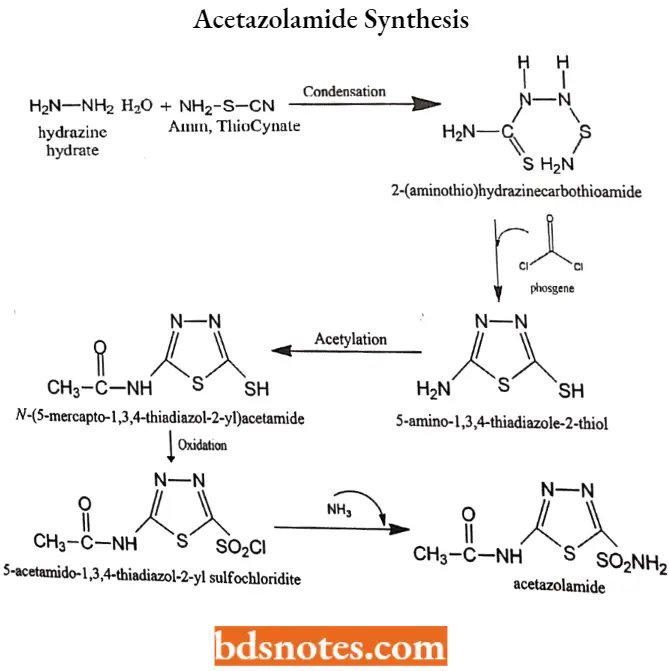
Acetazolamide Synthesis:
“How do diuretics work at the molecular level? FAQ answered”
Methazolamide
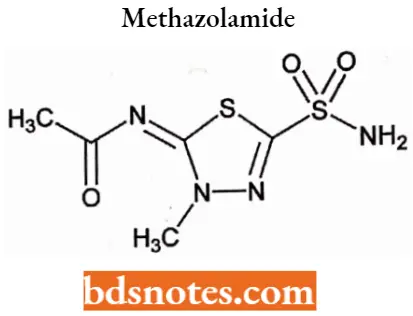
Methazolamide IUPAC name: N-(3-methyl-5-sulfamoyl-2,3-dihydro-l,3,4-thiadiazol-2-ylidene)acetamide.
Methazolamide MOA: Methazolamide is a potent inhibitor of carbonic anhydrase. Inhibition of carbonic anhydrase in the ciliary processes of the eye decreases aqueous humor secretion, presumably by slowing the formation of bicarbonate ions with subsequent reduction in sodium and fluid transport.
Methazolamide Uses: For treatment of chronic open-angle glaucoma and acute angle-closure glaucoma.
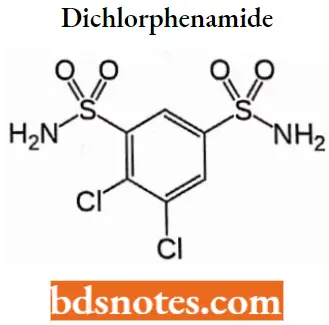
Dichlorphenamide
“Importance of studying medicinal chemistry of diuretics for pharmacology students: Questions explained”
Dichlorphenamide IUPAC name: 4,5-dichlorobenzene-l,3-disulfonamide.
Dichlorphenamide MOA: It reduces intraocular pressure by partially suppressing the secretion of aqueous humor (inflow), although the mechanism by which they do this is not fully understood.
- Evidence suggests that HCO3- ions are produced in the ciliary body by hydration of carbon dioxide under the influence of carbonic anhydrase and diffuse into the posterior chamber which contains more Na+ and HCO3- ions than dose plasma and consequently is hypertonic.
- Water is then attracted to the posterior chamber by osmosis, resulting in a pressure drop.
Dichlorphenamide Uses: For adjunctive treatment of chronic simple (OPen-angle) glaucoma, Secondary glaucoma, and preoperatively in acute angle-closure glaucoma where delay of surgery is desired to lower intraocular pressure.
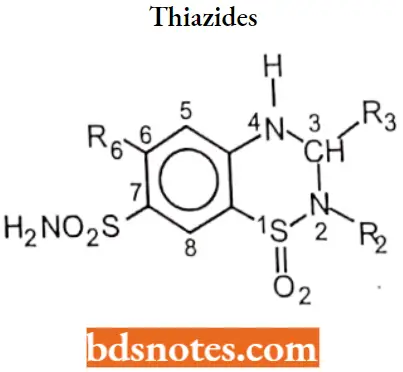
Thiazides
Thiazides are also called benzothiadiazides. Thiazides are sulfonamide derivatives.
Thiazides SAR:
“Common challenges in understanding diuretic mechanisms effectively: FAQs provided”
- H atom at N-2 is the most acidic due to the electron-withdrawing effects of the neighboring sulfone group.
- The sulfonamide group at C-7 provides an additional point of acidity in the molecule but is less acidic than the N-2 proton. A free sulfamoyl group at position 7 is essential for diuretic activity.
- These acidic protons make possible the formation of a water-soluble sodium salt that can be used for I.V. dosing.
- An electron-withdrawing group is essential at position 6.
- The diuretic activity is enhanced by substitution at position 3.
- Replacement of 6-Cl by 6-CF3 does not change potency but alters the duration of action.
- Replacement of 6-Cl by electron-donating groups (For Example. CH3) reduces diuretic activity.
- Saturation of thiadiazine ring to give 3, 4-dihydro derivative and replacement replace or removal of sulfonamide group at position C-7 yields compounds with little or no diuretic activity.
Thiazides MOA: Thiazides inhibit a Na+—Cl– symport in the luminal membrane of the epithelial cells in the distal convoluted tubule. Thus, thiazides inhibit NaCl reabsorption in the distal convoluted tubule and may have a small effect on the NaCl reabsorption in the proximal tubule.
Thiazides enhance Ca++ reabsorption in the distal convoluted tubule by inhibiting Na+ entry and thus enhancing the activity of Na+ —Ca++ exchanger in the basolateral membrane of epithelial cells.
Chlorothiazide
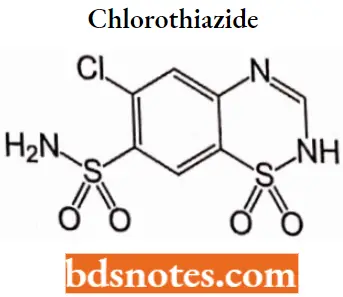
Chlorothiazide IUPAC name|6-chloro-l,l-dioxo4H-lX6,2,4-benzothiadiazine-7-sulfonamide.
Chlorothiazide MOA: As a diuretic, chlorothiazide inhibits active chloride reabsorption at the early distal tubule via the Na-Cl cotransporter, increasing the excretion of sodium, chloride, and water.
- Thiazides like chlorothiazide also inhibit sodium ion transport across the renal tubular epithelium through binding to the thiazide-sensitive sodium-chloride transporter.
- This results in an increase in potassium excretion via the sodium-potassium exchange mechanism.
- The antihypertensive mechanism of chlorothiazide is less well understood although it may be mediated through its action on carbonic anhydrases in the smooth muscle or through its action on the large-conductance calcium-activated potassium (KCa) channel, also found in the smooth muscle.
Chlorothiazide Uses: Chlorothiazide is indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy.
It is also indicated in the management of hypertension either as the sole therapeutic agent or to enhance the effectiveness of other antihypertensive drugs in the more severe forms of hypertension.
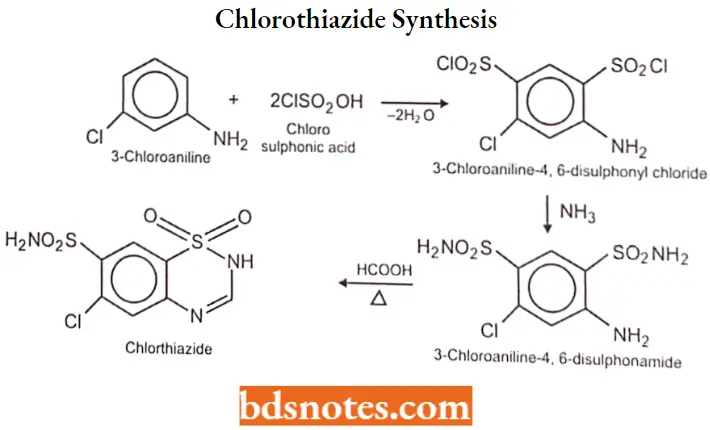
Chlorothiazide Synthesis:
“Why is early learning of diuretic medicinal chemistry critical for drug design? Answered”
Hydrochlorothiazide
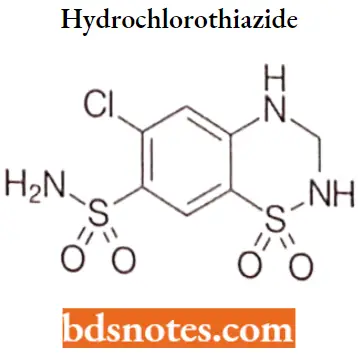
Hydrochlorothiazide IUPAC name: 6-chloro-1,1-dioxo-3,4-dihydro-2H-1λ6,2,4-benzothiadiazine-7-sulfonamicle.
Hydrochlorothiazide MOA: Hydrochlorothiazide, a thiazide diuretic, inhibits water reabsorption in the nephron by inhibiting the sodium-chloride symporter (SLC12A3) in the distal convoluted tubule, which is responsible for 5% of total sodium reabsorption.
- Normally, the sodium-chloride symporter transports sodium and chloride from the lumen into the epithelial cell lining the distal convoluted tubule.
- The energy for this is provided by a sodium gradient established by sodium-potassium ATPases on the basolateral membrane.
- Once sodium has entered the cell, it is transported out into the basolateral interstitium via the sodium-potassium ATPase, causing an increase in the osmolarity of the interstitium, thereby establishing an osmotic gradient for water reabsorption.
- By blocking the sodium-chloride symporter, hydrochlorothiazide effectively reduces the osmotic gradient and water reabsorption throughout the nephron.
Hydrochlorothiazide Uses: For the treatment of high blood pressure and management of edema.
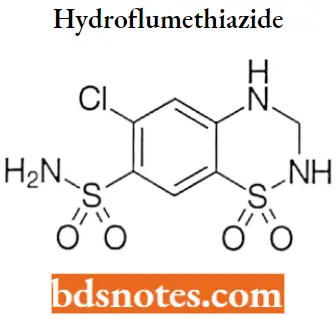
Hydroflumethiazide
“Factors influencing success with diuretic medicinal chemistry knowledge: Q&A”
Hydroflumethiazide IUPAC name: 1,1-dioxo-6-(trifluoromethyl)-3,4-dihydro-2H-1λ6,2,4-benzothiadiazine-7-sulfonamide.
Hydroflumethiazide MOA: Hydroflumethiazide is a thiazide diuretic that inhibits water reabsorption in the nephron by inhibiting the sodium-chloride symporter (SLC12A3) in the distal convoluted tubule, which is responsible for 5% of total sodium reabsorption.
Hydroflumethiazide Uses: Used as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy.
Also used in the management of hypertension either as the sole therapeutic agent or to enhance the effect of other antihypertensive drugs in the severe forms of hypertension.
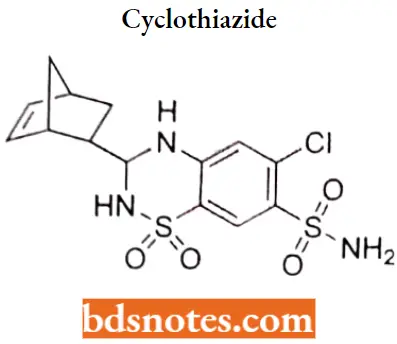
Cyclothiaride
“Differential applications of thiazide diuretics vs loop diuretics: Notes explained”
Cyclothiaride IUPAC name: 3-{bicyclo[2.2.1]hept-5-en-2-yl)-6-chloro-1,1-dioxo-3,4-dihydro-2H-1λ6,2,4-benzothiadiazine-7-sulfonamide.
Cyclothiaride MOA: As a diuretic, cydothiazide inhibits active chloride reabsorption at the early distal tubule via the Na-Cl cotransporter, increasing the excretion of sodium, chloride, and water.
- Thiazides like cydothiazide also inhibit sodium ion transport across the renal tubular epithelium by binding to the thiazide-sensitive sodium-chloride transporter.
- This results in an increase in potassium excretion via the sodium-potassium exchange mechanism.
- The antihypertensive mechanism of cydothiazide is less well understood although it may be mediated through its action on carbonic anhydrases in the smooth muscle or through its action on the large-conductance calcium-activated potassium (KCa) channel, also found in the smooth muscle.
CyclothiarideUses: Cydothiazide is indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy.
It is also indicated in the management of hypertension either as the sole therapeutic agent or to enhance the effectiveness of other antihypertensive drugs in the more severe forms of hypertension.
Loop diuretics
The diuretics that produce peak diuresis than other diuretics and act distinctly on renal tubular function (at the loop of Henle) are called loop diuretics or high-ceiling diuretics. There are two major classes of loop diuretics
- Sulfonamide derivatives such as furosemide, bumetanide and torsemide.
- Non-sulfonamide loop diuretic such as ethacrynic acid.
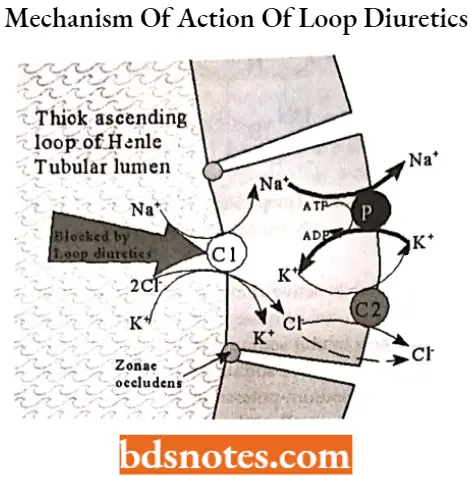
Loop diuretics MOA: Loop diuretics inhibit the reabsorption of NaCl and KCl by inhibiting the Na+ —K+ —2Cl– symport in the luminal membrane of the thick ascending limb (TAL) of the loop of Henle.
- As TAL is responsible for the reabsorption of 35% of filtered sodium, loop diuretics are highly efficacious and are thus called high-ceiling diuretics.
- The Na* —K+ —20“ symport and sodium pump together generate a positive lumen potential that drives the reabsorption of Ca++ and Mg++, inhibitors of the Na+ -K+ -2CI– symport also inhibit the reabsorption of Ca++ and Mg++.
- Loop diuretics also have direct effects on vasculature including an increase in renal blood flow and an increase in systemic venous capacitance.
“Steps to explain types of diuretics: Loop diuretics vs thiazides vs potassium-sparing diuretics: Notes guide”
Loop diuretics SAR:
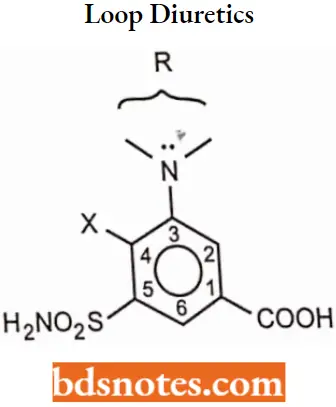
- The substituent at 1-position must be acidic, the carboxyl group provides optimal diuretic activity, but other groups as tetrazole, may have diuretic activity.
- A sulfamoyl group in the 5-position is essential for optimal high-ceiling diuretic activity.
- The activating group (X-) in the 4- position can be Cl– or CF3-, a phenoxy-, alkoxy-, aniline-, benzyl-, orbenzoyl- group.
- Substituents that can be tolerated on the 2-amino group series only furfuryl- >benzyl>thienylmethy.
- substituent, on the 3-amino group series, can vary widely without affecting optimal diuretic activity.
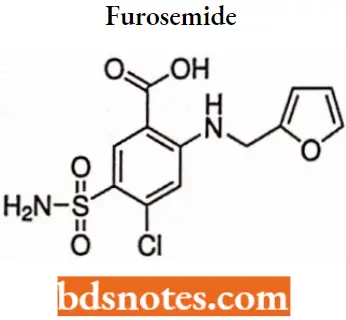
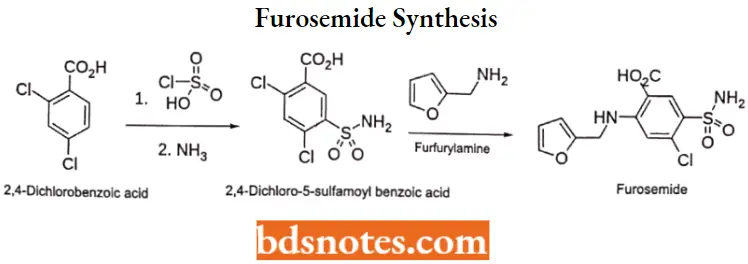
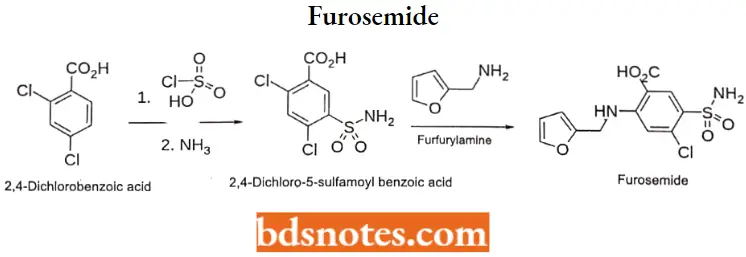
Furosemide
“Role of Na-K-Cl symport inhibition in loop diuretics: Questions answered”
Furosemide IUPAC name: 4-chloro-2-[(furan-2-ylmethyl)ainino]-5-sulfamoylbenzoic add.
Furosemide MOA: Furosemide, a loop diuretic, inhibits water reabsorption in the nephron by blocking the sodium potassium-chloride cotransporter (NKCC2) in the thick ascending limb of the loop of Henle.
- This is achieved through competitive inhibition at the chloride binding site on the cotransporter, thus preventing the transport of sodium from the lumen of the loop of Henle into the basolateral interstitium.
- Consequently, the lumen becomes more hypertonic while the interstitium becomes less hypertonic, which in turn diminishes the osmotic gradient for water reabsorption throughout the nephron.
- Because the thick ascending limb is responsible for 25% of sodium reabsorption in the nephron, furosemide is a very potent diuretic.
Furosemide Metabolism: Only a small amount is hepatically metabolized to the defurfurylated derivative, 4-chloro-5- sulfamoyl anthranilic add.
Furosemide Uses: For the treatment of edema associated with congestive heart failure, cirrhosis of the liver, and renal disease, including nephrotic syndrome.
Also for the treatment of hypertension alone or in combination with other antihypertensive agents.
Furosemide Synthesis:
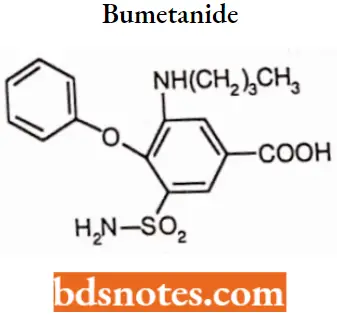
Bumetanide
“How does furosemide differ from hydrochlorothiazide? FAQ explained”
Bumetanide IUPAC name: 3-(butylamino)-4-phenoxy-5-sulfamoylbenzoic acid.
Bumetanide MOA: Bumetanide interferes with renal cAMP and/or inhibits the sodium-potassium ATPase pump. Bumetanide appears to block the active reabsorption of chloride and possibly sodium m the ascending loop of Henle, altering electrolyte transfer in the proximal tubule. This results in the excretion of sodium, chloride, and water and, hence, diuresis.
Bumetanide Uses: For the treatment of edema associated with congestive heart failure and hepatic and renal disease including nephrotic syndrome.
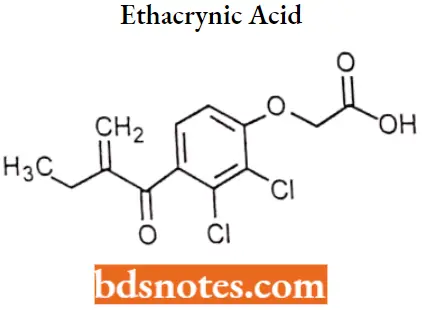
Ethacrynic acid
“Asymptomatic vs symptomatic effects of outdated diuretic practices: Answered”
Ethacrynic acid IUPAC name: 2-[2,3-dichloro-4-(2-methylidenebutanoyl)phenoxy]acetic acid.
Ethacrynic acid MOA: Ethacrynic acid inhibits the symport of sodium, potassium, and chloride primarily in the ascending limb of Henle, but also in the proximal and distal tubules.
- This pharmacological action results in the excretion of these ions, increased urinary output, and reduction in extracellular fluid Diuretics also lower blood pressure initially by reducing plasma and extracellular fluid volume; cardiac output also decreases, explaining its antihypertensive action.
- Eventually, cardiac output returns to normal with an accompanying decrease in peripheral resistance. Its mode of action does not involve carbonic anhydrase inhibition.
Ethacrynic acid Uses: For the treatment of high pressure and edema caused by diseases like congestive heart failure, liver, and kidney failure.
Potassium-sparing diuretics
Potassium-sparing diuretics do not share any obvious chemical similarities, except for the steroidstructure of the aldosterone antagonists. Those in clinical use include:
Aldosterone antagonists (mostly spironolactone)- Spironolactone, Eplerenone. Epithelial sodium channel blockers- Amiloride, Triamterene.
Aldosterone antagonists
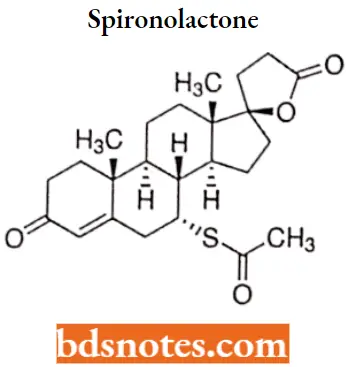
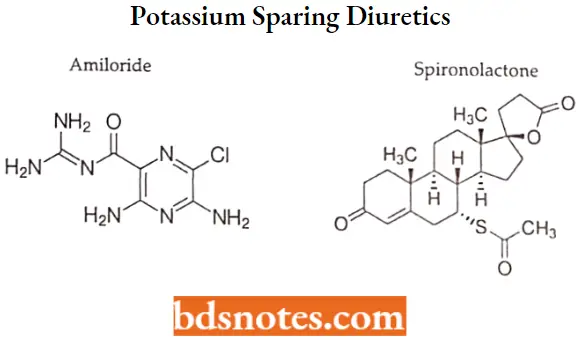
Spironolactone
“Early warning signs of gaps in understanding diuretic SAR basics: Common questions”
Spironolactone IUPAC name: (1’S, 2’R,2’R,9’R,10,R,11’S,15’S)-9′-(acetylsulfanyl)-2′,15’-dimethylspiro[oxolane-2,14′- tetracycloheptadecan]-6’-ene-5,5′-dione.
Spironolactone MOA: Spironolactone is a specific pharmacologic antagonist of aldosterone, acting primarily through competitive binding of receptors at the aldosterone-dependent sodium-potassium exchange site in the distal convoluted renal tubule.
Spironolactone causes increased amounts of sodium and water to be excreted, while potassium is retained. Spironolactone acts both as a diuretic and as an antihypertensive drug by this mechanism.
Spironolactone Uses: Used primarily to treat low-renin hypertension, hypokalemia, and Conn’s syndrome.
Epithelial sodium channel blockers
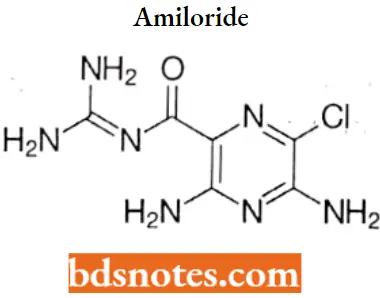
Amiloride
“Asymptomatic vs symptomatic effects of ignoring diuretic principles: Q&A”
Amiloride IUPAC name: 3,5-diamino-6-chloro-N-(diaminomethylidene)pyrazine-2-carboxamide.
Amiloride MOA: Amiloride works by inhibiting sodium reabsorption in the distal convoluted tubules and collecting ducts in the kidneys by binding to the amiloride-sensitive sodium channels.
- This promotes the loss of sodium and water from the body, but without depleting potassium. Amiloride exerts its potassium-sparing effect through the inhibition of sodium reabsorption at the distal convoluted tubule.
- Cortical collecting tubule and collecting duct; this decreases the net negative potential of the tubular lumen and reduces both potassium and hydrogen secretion and their subsequent excretion.
- Amiloride is not an aldosterone antagonist and its effects are seen even in the absence of aldosterone.
Amiloride Uses: For use as adjunctive treatment with thiazide diuretics or other kaliuretic-diuretic agents in congestive heart failure or hypertension.
Triamterene
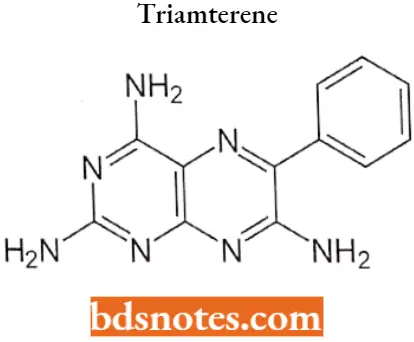
Triamterene IUPAG name: 6-phenylpteridine-2,4,7-triamine.
Triamterene MOA: Triamterene inhibits the epithelial sodium channels on principal cells in the late distal convoluted tubule and collecting tubule, which are responsible for 1-2% of total sodium reabsorption.
- As sodium reabsorption is inhibited, this increases the osmolarity in the nephron lumen and decreases the osmolarity of the interstitium.
- Since sodium concentration is the main driver for water reabsorption, triamterene can achieve a modest amount of diuresis by decreasing the osmotic gradient necessary for water reabsorption from the lumen to the interstitium.
- Triamterene also has a potassium-sparing effect. Normally, the process of potassium excretion is driven by the electrochemical gradient produced by sodium reabsorption.
- As sodium is reabsorbed, it leaves a negative potential in the lumen, while producing a positive potential in the principal cell. This potential promotes potassium excretion through apical potassium channels.
- By inhibiting sodium reabsorption, triamterene also inhibits potassium excretion.
Triamterene Uses: For the treatment of edema associated with congestive heart failure, cirrhosis of the liver, and nephrotic syndrome; also in steroid-induced edema, idiopathic edema, and edema due to secondary hyperaldosteronism.
Osmotic diuretics
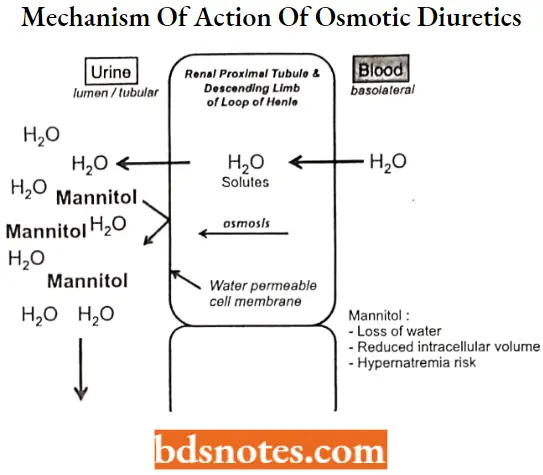
Osmotic diuretics are the agents that mobilize fluids by increasing the osmotic pressure in tubules.
Osmotic diuretics MOA:
“Can targeted interventions improve outcomes using diuretic medicinal chemistry knowledge? FAQs provided”
- Osmotic diuretics are substances to which the tubule epithelial cell membrane has limited permeability. When administered (often in a large dosage), osmotic diuretics significantly increase the osmolarity of plasma and tubular fluid.
- The osmotic force thus generated prevents water reabsorption, and also extracts water from the intracellular compartment, expands extracellular fluid volume, and increases renal blood flow resulting in reduced medulla tonicity.
- The primary sites of action for osmotic diuretics are the Loop of Henle and the proximal tubule where the membrane is most permeable to water.
Mannitol
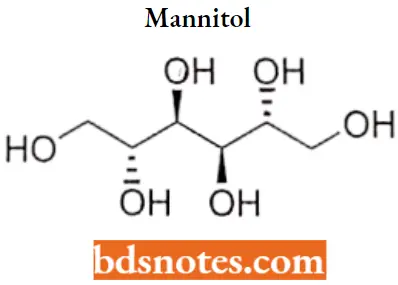
Mannitol IUPAC name: (2R,3R,4R,5R)-hexane-1,2,3,4,5,6-hexol.
Mannitol MOA: As a diuretic mannitol induces diuresis because it is not reabsorbed in the renal tubule, thereby increasing the osmolality of the glomerular filtrate, facilitating excretion of water, and the renal tubular reabsorption of sodium, chloride, and other solutes.
- Mannitol promotes the urinary excretion of toxic materials and protects against nephrotoxicity by preventing the concentration of toxic substances in the tubular fluid.
- As an Antiglaucoma agent mannitol elevates blood plasma osmolarity, resulting in enhanced flow of water from the eye into plasma and a consequent reduction in intraocular pressure.
Mannitol Uses:
Used for the promotion of diuresis before irreversible renal failure becomes established, the reduction of intracranial pressure, the treatment of cerebral edema, and the promotion of urinary excretion of toxic substances.
Adverse Effects Of Diuretics
Loop and thiazide diuretics can cause metabolic alkalosis due to increased excretion of chloride in proportion to bicarbonate. This is more common with loop diuretics than thiazide diuretics.
- They can also cause hypokalemia, hyperglycemia and glucose intolerance, hyperlipidemia, hyponatremia, hyperuricemia, and hypomagnesemia. Thiazide diuretics can cause hypercalcemia while loop diuretics increase the excretion of calcium which can lead to hypocalcemia.
- Moreover, loop and thiazide diuretics are sulfonamides and can lead to allergic reactions. Loop diuretics also have the potential to cause ototoxicity and hearing loss. Of note, hypokalemia can cause ventricular arrhythmias and muscular weakness.
- Spironolactone’s primary adverse effect is hyperkalemia, especially in elderly patients and those with chronic kidney disease.
- Individuals at higher risk for hyperkalemia also include those on beta blockers, ACE inhibitors, angiotensin receptor blockers, and NSAIDs. It also has antiandrogenic effects, including gynecomastia, hirsutism, and sexual dysfunction.
- Acetazolamide is a sulfonamide and can lead to allergic reactions. Other adverse reactions include paresthesias, tinnitus, taste alteration, and metabolic acidosis (especially in the elderly and kidney disease.
Mannitol has complex effects on electrolytes. It initially leads to hypertonic hyponatremia when it recruits water from cells. Later as the extracellular fluid is excreted, hyperkalemic acidosis can develop. After this hypernatremic dehydration may occur.
“Differential applications of diuretics in hypertension vs edema: Notes explained”
Diuretics Multiple Choice Questions And Answers
Question 1. Which of these beverages does not have a diuretic effect?
- Alcohol
- Milk
- Tea
- Coffee
Answer: 2. Milk
Question 2. Progesterone
- ADH
- ANH
- Aldosterone
- PTH
Answer: 3. Aldosterone
Question 3. Side effects for this particular diuretic include hyperkalemic metabolic acidosis and gynecomastia.
- Amiloride
- Hydrochlorothiazide
- Furosemide
- Spironolactone
Answer: 4. Spironolactone
Question 4. One of the most powerful “high ceiling” diuretics that has a short duration of action, inhibits the Na/K/Cl transporter and can block the reabsorption of up to 30% of filtered sodium.
- Acetazolamide
- Hydrochlorothiazide
- Ethacrynic acid
- Spironolactone
Answer: 3. Ethacrynic acid
“Steps to apply diuretic medicinal chemistry in drug discovery: Design vs optimization: Notes guide”
Question 5. A drug that acts on the proximal tubule has a relatively low efficacy in blocking the reabsorption of Na but is useful in the treatment of glaucoma, and as a prophylactic to prevent acute mountain sickness.
- Acetazolamide
- Triamterene
- Ethacrynic acid
- Furosemide
Answer: 1. Acetazolamide
Question 6. A drug with modest effects that is often reserved for use in patients who develop hypokalemia in response to taking another more potent diuretic.
- Ethacrynic add
- Indapamide
- Triamterene
- Furosemide
Answer: 3. Triamterene
Question 7. Which drug causes an increase in plasma glucose, urate levels, and lipid levels?
- Mannitol
- Hydrochlorothiazide
- Spironolactone
- Furosemide
Answer: 2. Hydrochlorothiazide
Question 8. Which drug increases the risk of kidney stones? Decreases?
- Furosemide, Spironolactone
- Furosemide, Hydrochlorothiazide
- Hydrochlorothiazide, Furosemide
- Hydrochlorothiazide, Spironolactone
Answer: 2. Furosemide, Hydrochlorothiazide
Question 9 Which of the following drugs is paradoxically used to treat Diabetes insipidus?
- Hydrochlorothiazide
- Mannitol
- Spironolactone
- Furosemide
Answer: 1. Hydrochlorothiazide
“Role of SAR in improving diuretic efficacy: Questions answered”
Question 10. Which type of drug can pull water out of cells all over the body?
- Mannitol
- Amiloride
- Thiazide diuretics
- Acetazolamide
Answer: 1. Mannitol
Diuretics Short Questions And Answers
Question 1. Discuss mode of action of thiazides.
Answer:
Thiazides inhibit a Na+—Cl– symport in the luminal membrane of the epithelial cells in the distal convoluted tubule. Thus, thiazides inhibit NaCl reabsorption in the distal convoluted tubule and may have a small effect on the NaCl reabsorption in the proximal tubule.
Thiazides enhance Ca++ reabsorption in the distal convoluted tubule by inhibiting Na+ entry and thus enhancing the activity of Na+ —Ca++ exchanger in the basolateral membrane of epithelial cells.
Question 2. Outline synthesis and clinical Use of Furosemide.
Answer:
Synthesis
“How does spironolactone act as a potassium-sparing diuretic? FAQ explained”
Clinical uses: For the treatment of edema associated with congestive heart failure, cirrhosis of the liver, and renal disease, including nephrotic syndrome.
Also for the treatment of hypertension alone or in combination with other antihypertensive agents.
Question 3. Write MOA of osmotic diuretics.
Answer:
Osmotic diuretics are substances to which the tubule epithelial cell membrane has limited permeability. When administered (often in a large dosage), osmotic diuretics significantly increase the osmolarity of plasma and tubular fluid.
- The osmotic force thus generated prevents water reabsorption, and also extracts water from the intracellular compartment, expands extracellular fluid volume, and increases renal blood flow resulting in reduced medulla tonicity.
- The primary sites of action for osmotic diuretics are the Loop of Henle and the proximal tubule where the membrane is most permeable to water.
“Early warning signs of complications from ignoring diuretic protocols: Common questions”
Question 4. Draw the structure of any two potassium-sparing diuretics.
Answer:
Leave a Reply