Antineoplastic Drugs Introduction
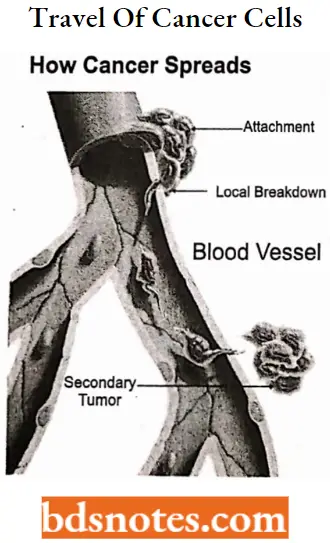
Cancer is the rapid creation of abnormal cells that grow beyond their usual boundaries, and which can then invade adjoining parts of the body and spread to other organs.
- This process is referred to as metastasis. Metastases are the major cause of death from cancer. (WHO).
- Cancer known medically as a malignant neoplasm is a broad group of diseases involving unregulated cell growth.
- In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invading nearby parts of the body through the lymphatic system or bloodstream.
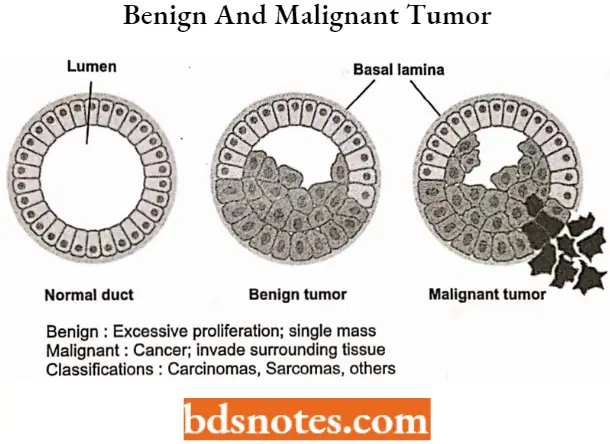
Not all tumors are cancerous; benign tumors do not invade neighboring tissues and do not spread throughout the body. There are over 200 different known cancers that affect humans.
Read and Learn More Medicinal Chemistry II Notes
“Understanding antineoplastic agents through FAQs: Medicinal chemistry explained”
Benign Tumor
A benign tumor is not a cancerous tumor. Unlike cancer tumors, a non-cancerous tumor is unable to spread throughout the body.
- A non-malignant tumor can be serious if they are pressing a primary nerve, a main artery, or compresses brain matter. Overall, benign tumors respond well to treatment and the prognosis is usually favorable.
- Some suspected causes of benign tumors include a traumatic injury at the tumor location, chronic inflammation (or long-term stress that leads to inflammation), an undetected infection, or diet.
Malignant Tumor
The word malignant is Latin for “badly bom.” This type of tumor can multiply uncontrollably, metastasize (spread) to various parts of the body and invade surrounding tissue.
- Malignant tumors are formed from abnormal cells that are highly unstable and travel via the bloodstream, circulatory system, and lymphatic system.
- Malignant cells do not have chemical adhesion molecules to anchor them to the original growth site that benign tumors possess.
- There are many suspected causes of cancer- some are widely accepted by the medical community while others are not.
obesity, smoking, alcohol consumption, poor diet, environmental pollution, heavy metal exposure, and household toxins are a few culprits that may lead to cancer in your body.
“How do antineoplastic drugs target cancer cells? FAQ answered”
Cancers are often described by the body part that they originated in. However, some body parts contain multiple types of tissue, so for greater precision, cancers are additionally classified by the type of cell that the tumor cells originated from. These types include:
Types Of Cancer
Carcinoma
Cancers derived from epithelial cells. This group includes many of the most common cancers, particularly in older adults. Nearly all cancers developing in the breast, prostate, lung, pancreas, and colon are carcinomas.
Sarcoma
Cancers arise from connective tissue (i.e. bone, cartilage, fat, nerve), each of which develops from cells originating in mesenchymal cells outside the bone marrow.
Lymphoma and leukemia
These two classes of cancer arise from cells that make blood. Leukemia is the most common type of cancer in children accounting for about 30%. However, far more adults develop lymphoma and leukemia.
Germ cell tumor
Cancers are derived from pluripotent cells, most often presenting in the testicle or the ovary (seminoma and dysgerminoma, respectively).
Blastoma
Cancers are derived from immature “precursor” cells or embryonic tissue. Blastomas are more common in children than in older adults.
Cell Cycle In Cancer
The cell cycle, the process by which cells progress and divide, lies at the heart of cancer.
- In normal cells, the cell cycle is controlled by a complex series of signaling pathways by which a cell grows, replicates its DNA, and divides.
- This process also includes mechanisms to ensure errors are corrected, and if not, the cells commit suicide (apoptosis).
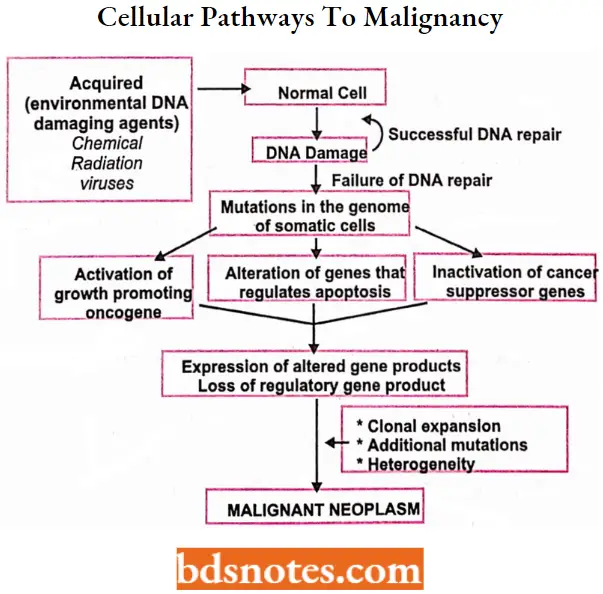
- In cancer, as a result of genetic mutations, this regulatory process malfunctions, resulting in uncontrolled cell proliferation.
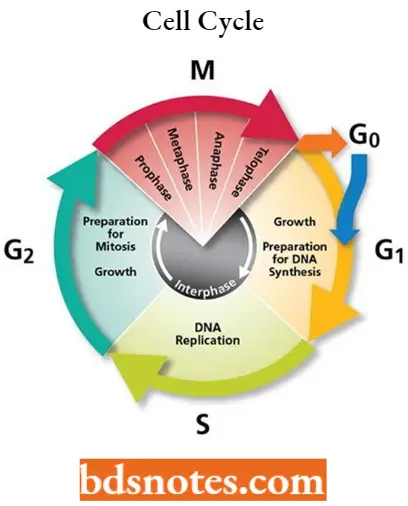
The cell cycle involves a complex series of molecular and biochemical signaling pathways. As illustrated in the diagram below, the cell cycle has four phases:
“Importance of studying medicinal chemistry of antineoplastic drugs for pharmacology students: Questions explained”
- The G1, or gap, phase, is in which the cell grows and prepares to synthesize DNA.
- The S, or synthesis, phase, in which the cell synthesizes DNA.
- The G2, or second gap, phase, in which the cell prepares to divide.
- The M, or mitosis, phase, in which cell division occurs.
As a cell approaches the end of the G1 phase it is controlled at a vital checkpoint, called G1/S, where the cell determines whether or not to replicate its DNA.
- At this checkpoint, the cell is checked for DNA damage to ensure that it has all the necessary cellular machinery to allow for successful cell division.
- As a result of this check, which involves the interactions of various proteins, a “molecular switch” is toggled on or off.
- Cells with intact DNA continue to S phase; cells with damaged DNA that cannot be repaired are arrested and “commit suicide” through apoptosis, or programmed cell death.
- A second such checkpoint occurs at the G2 phase following the synthesis of DNA in the S phase but before cell division in the M phase. Cells use a complex set of enzymes called kinases to control various steps in the cell cycle.
Cyclin-dependent kinases, or CDKs, are a specific enzyme family that uses signals to switch on cell cycle mechanisms.
- CDKs themselves are activated by forming complexes with cyclins, another group of regulatory proteins only present for short periods in the cell cycle.
- When functioning properly, cell cycle regulatory proteins act as the body’s tumor suppressors by controlling cell growth and inducing the death of damaged cells.
- Genetic mutations causing the malfunction or absence of one or more of the regulatory proteins at cell cycle checkpoints can result in the “molecular switch” being turned permanently on, permitting uncontrolled multiplication of the cell, leading to carcinogenesis, or tumor development.
Cellular Pathways To Malignancy
“Common challenges in understanding antineoplastic drug mechanisms effectively: FAQs provided”
Classification Of Chemotherapeutic Agents
Classification according to phase-specific toxicity
- Cytotoxic drugs can be classified according to whether they are more likely to target cells in a particular phase of their growth cycle.
- More crudely, they can also be divided into whether they are more toxic to cells that are actively dividing rather than cells in both the proliferating and resting phases.
Phase-specific Chemotherapy
These drugs, such as methotrexate and vinca alkaloids, kill proliferating cells only during a specific part or part of the cell cycle.
- Antimetabolites, such as methotrexate, are more active against S-phase cells (inhibiting DNA synthesis) whereas vinca alkaloids are more M-phase specific (inhibiting spindle formation and alignment of chromosomes).
- Attempts have been made to time drug administration in such a way that the cells are synchronized into a phase of the cell cycle that renders them especially sensitive to the cytotoxic agent.
- For example, vinblastine can arrest cells in mitosis. These synchronized cells enter the S-phase together and can be killed by a phase-specific agent, such as cytosine arabinoside. Most current drug schedules, however, have not been devised based on cell kinetics.
Cell Cycle-specific Chemotherapy
Most chemotherapy agents are cell cycle-specific, meaning that they act predominantly on cells that are actively dividing.
- They have a dose-related plateau in their cell-killing ability because only a subset of proliferating cells remains fully sensitive to drug-induced cytotoxicity at any one time.
- The way to increase cell kill is therefore to increase the duration of exposure rather than increasing the drug dose.
Cell Cycle-nonspecific Chemotherapy
These drugs, for example, alkylating agents and platinum derivatives, have an equal effect on tumor and normal cells whether they are in the proliferating or resting phase.
They have a linear dose-response curve; that is, the greater the dose of the drug, the greater the fractional cell kill.
“Why is early learning of antineoplastic medicinal chemistry critical for cancer treatment? Answered”
Chemotherapeutic Agents Classification
According to Drug Acting Directly on Cells
- Alkylating agents
- Nitrogen Mustard For Example. Mechlorethamine (Mustine HCL), Cyclophosphamide, Ifosfamide, Chlorambucil.
- Ethylenimine For Example. Thio- TEPA.
- Alkyl Sulphonate For Example. Busulfan.
- Nitrosourea For Example. Carmustine, Lomustine.
- Triazine For Example. Dacarbazine.
- Antimetabolites
- Folate Antagonist For Example. Methotrexate.
- Purine Antagonist For Example. 6-Mercaptopurine, 6-thiogunine, Azathioprine.
- Pyrimidine Antagonist For Example. 5-Fluorouracil, Cytarabine.
- Vinka Alkaloids For Example. Vincristine, Vinblastine.
- Taxanes For Example. Paclitaxel.
- Epipodophyllotoxin For Example. Etoposide.
- Antibiotics For Example. Actinomycin, Doxorubicin, Daunorubicin, Mitoxantrone, Bleomycins, Mithramycin.
- Miscellaneous For Example. Hydroxyurea, Procarbazine, L- Asparaginase, Cisplatin, and Carboplatin.
“Factors influencing success with antineoplastic drug knowledge: Q&A”
According to Drug Acting Directly on Cells
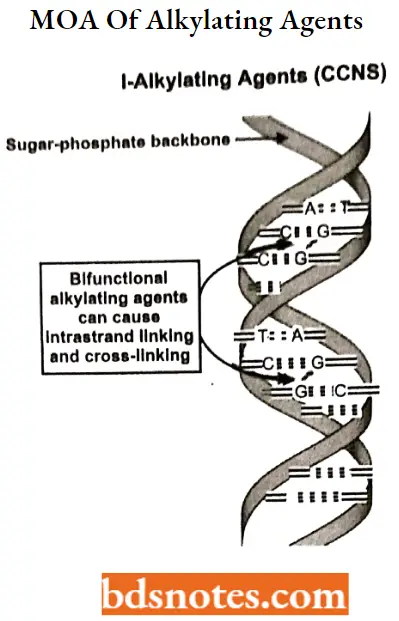
Alkylating agents: Alkylating agents involve reactions with guanine in DNA. These drugs add methyl or other alkyl groups onto molecules where they do not belong. This in turn inhibits their correct utilization by base pairing and causes a miscoding of DNA.
Alkylating agents MOA: In the first mechanism, an alkylating agent attaches alkyl groups to DNA bases. This alteration results in the DNA being fragmented by repair enzymes in their attempts to replace the alkylated bases.
- A second mechanism by which alkylating agents cause DNA damage is the formation of cross-bridges, bonds between atoms in the DNA.
- In this process, two bases are linked together by an alkylating agent that has two DNA binding sites. Cross-linking prevents DNA from being separated for synthesis or transcription.
- The third mechanism of action of alkylating agents causes the mispairing of the nucleotides leading to mutations.
- There are six groups of alkylating agents: nitrogen mustards; ethylene; alkylsulfonates; triazenes; piperazines; and nitrosureas.
“Steps to explain types of antineoplastic drugs: Alkylating agents vs antimetabolites vs topoisomerase inhibitors: Notes guide”
1. Nitrogen Mustards
Cyclophosphamide
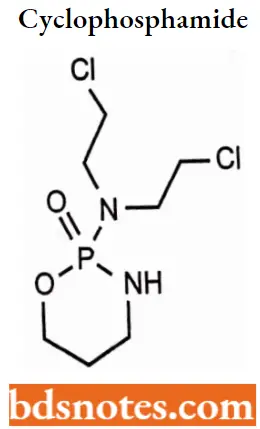
Cyclophosphamide IUPAC name: (RS)-N, N-bis(2-chloroethyl)-l,3,2-oxazaphosphinan-2-amine 2-oxide.
Cyclophosphamide MOA: The main effect of cyclophosphamide is due to its metabolite phosphoramide mustard. This metabolite is formed in cells that have low levels of ALDH.
- Phosphoramide mustard forms DNA crosslinks both between and within DNA strands at guanine N-7 positions (known as interstrand and intrastrand cross-linkages, respectively). This is irreversible and leads to cell apoptosis.
- Cyclophosphamide has relatively little typical chemotherapy toxicity as ALDHs are present in relatively large concentrations in bone marrow stem cells, liver, and intestinal epithelium.
- ALDHs protect these actively proliferating tissues against the toxic effects of phosphoramide mustard and acrolein by converting aldophosphamide to carboxy cyclophosphamide that does not give rise to the toxic metabolites phosphoramide mustard and acrolein.
This is because carboxy cyclophosphamide cannot undergo p-elimination (the carboxylate acts as an electron-donating group, forbidding the transformation), preventing nitrogen mustard activation and subsequent alkylation
- Cyclophosphamide induces beneficial immunomodulatory effects in adaptive immunotherapy.
- Suggested mechanisms include:
- Elimination of T regulatory cells (CD4+CD25+ T cells) in native and tumor-bearing host cells,
- Induction of T cell growth factors, such as type 1 IFNs,
- Thus, cyclophosphamide preconditioning of recipient hosts (for donor T cells) has been used to enhance immunity in native hosts and to enhance adoptive T cell immunotherapy regimens, as well as active vaccination strategies, Indicating objective antitumor immunity.
“Role of DNA alkylation in antineoplastic drug action: Questions answered”
Cyclophosphamide SAR: Bis-2-chloroethyl amino group is essential for antineoplastic activity,
- Chloro atom provides maximum activity
- Cyclophosphamide is chemically related to nitrogen mustards, the nucleophilicity of the mustard nitrogen is substantially reduced through an amide-like phosphoramide linkage and hence less likely to form aziridinium ion.
- Triethylene derivatives remain inactive,
- The levo form has twice the therapeutic index of the dextro form.
Cyclophosphamide Metabolism: Oral cyclophosphamide is rapidly absorbed and then converted by mixed-function oxidase enzymes (cytochrome P450 system) in the liver to active metabolites.
Cyclophosphamide metabolites are primarily excreted in the urine unchanged, and drug dosing should be appropriately adjusted in the setting of renal dysfunction.
Cyclophosphamide Therapeutic Uses: Cyclophosphamide is used as chemotherapy and to suppress the immune system.
- As chemotherapy, it is used to treat lymphoma, multiple myeloma, leukemia, ovarian cancer, breast cancer, small cell lung cancer, neuroblastoma, and sarcoma.
- As an immune suppressor it is used in nephrotic syndrome, granulomatosis with polyangiitis, and following organ transplant, other conditions.
Cyclophosphamide Adverse Reaction: Common side effects include low white blood cell counts, loss of appetite, vomiting, hair loss, and bleeding from the bladder.
Other severe side effects include an increased future risk of cancer, infertility, allergic reactions, and pulmonary fibrosis.
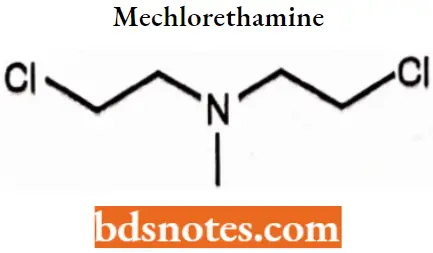
Mechlorethamine
“How does methotrexate inhibit cancer cell growth? FAQ explained”
Mechlorethamine IUPAC name: 2-Chloro-N-(2-ehloroethyl)-N-methylethan-1-amine,
Mechlorethamine MOA: It is the prototype of alkylating agents, a group of anticancer chemotherapeutic drugs, It works by binding to DNA, crosslinking two strands, and preventing cell duplication, It binds to the N7 nitrogen on the DNA base guanine.
Mechlorethamine SAR:
- B is-2-chloroethyl amino group is essential for antineoplastic activity.
- Modification of this group changes, stability, reactivity, and lipophilicity.
Mechlorethamine Metabolism: Undergoes rapid chemical transformation and combines with water or reactive compounds of cells, so that the drug is no longer present in active form a few minutes after administration.
Mechlorethamine Therapeutic Uses: It has been derivatized into the estrogen analog estramustine phosphate, used to treat prostate cancer.
- It can also be used in chemical warfare, Some uses of mechlorethamine have included lymphoid malignancies such as Hodgkin’s disease, lymphosarcoma, and chronic myelocytic leukemia.
- Polycythemia vera and bronchogenic carcinoma Mechlorethamine is often administered intravenously, but when compounded into a topical formulation it can also be used to treat skin diseases.
Mechlorethamine Adverse Reaction: Mechlorethamine is a highly toxic medication, especially for women who are pregnant, breastfeeding, or of childbearing age. At high enough levels, exposure can be fatal.
- It can cause immunosuppression and damage to mucous membranes of the eyes, skin, and respiratory tract.
- Mucous membranes and damp or damaged skin, Eye exposure causes lacrimation, burning, irritation, itching, a feeling of grittiness or dryness, blepharospasm (spasms of the eyelid), and miosis (pinpoint pupils).
- More severe cases cause edema (swelling from fluid accumulation) in the eyelids, photophobia (extreme sensitivity to light), severe pain, corneal ulceration, and blindness.
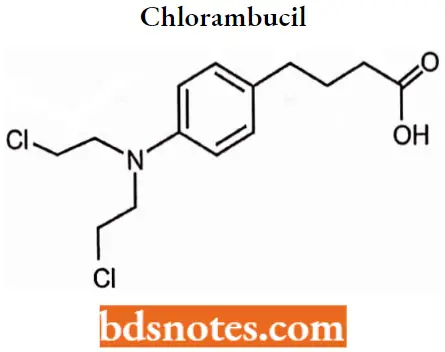
Chlorambucil
“Early warning signs of gaps in understanding antineoplastic SAR basics: Common questions”
Chlorambucil IUPAC name: 4-[bis(2-chlorethyl)amino]benzenebutanoic acid.
Chlorambucil MOA: Chlorambucil produces its anti-cancer effects by interfering with DNA replication and damaging the DNA in a cell. The DNA damage induces cell cycle arrest and cellular apoptosis.
Chlorambucil SAR: An aromatic N substituent (Phenyl) group conjugated with the mustard nitrogen will stabilize the lone pair of electrons through resonance. Aromatic mustards have fewer side effects
- Electron donating substituent on phenyl ring increases the activity of the drug.
- Those compounds having electron-withdrawing substituents don’t alkylate under physiological conditions and hence are less effective.
- Attempts were also made to get favorable activity by varying halogen atoms. Iodo derivatives generally exhibit low activity.
Chlorambucil Metabolism: Chlorambucil is extensively metabolized in the liver primarily to phenylacetic acid mustard.
The pharmacokinetic data suggests that oral chlorambucil undergoes rapid gastrointestinal absorption and plasma clearance and that it is almost completely metabolized, having extremely low urinary excretion.
Chlorambucil Therapeutic Uses: Chlorambucil was approved for medical use in the United States in 1957.
- It is on the World Health Organization’s List of Essential Medicines. Chlorambucil is used to treat chronic lymphocytic leukemia (CLL).
- Hodgkin’s disease, non-Hodgkin’s lymphoma, breast, ovarian and testicular cancer, Waldenstrom’smacroglobulinemia, thrombocythemia, choriocarcinoma.
Chlorambucil Adverse reaction: Common side effects include bone marrow suppression. Other serious side effects include an increased long-term risk of further cancer, infertility, and allergic reactions. Use during pregnancy often results in harm to the baby.
2. Alkyl sulphonate
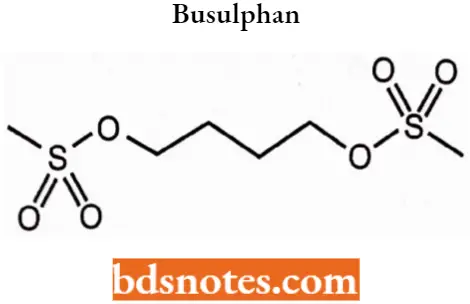
Busulphan
“Asymptomatic vs symptomatic effects of ignoring antineoplastic principles: Q&A”
Busulphan IUPAC name: Butane-1,4-diyl dimethanesulfonate.
Busulphan MOA: Busulfan is a bifunctional alkylating agent. Following systemic absorption,carbonium ions are rapidly formed, resulting in alkylation of DNA.
- This leads to breaks in DNA molecules as well as cross-linking of the twin strands resulting in interference of DAN replication and transcription of RNA replication.
- Because the intrastrand DNA crosslinks cannot be repaired by cellular machinery, the cell undergoes apoptosis.
Busulphan SAR:
- Dimethanesulfonate analogs of mustard were found to be effective.
- Symmetrical nature of drugs ( bifunctionality) results in increased activity.
Busulphan Metabolism: Busulfan is highly lipophilic and easily crosses the cell membrane. It is extensively metabolized and less than 2% is excreted unchanged.
Membrane transporters for busulfan have not been elucidated. Busulfan is primarily metabolized in the liver by glutathione s-transferases (GSTs).
Busulphan Therapeutic Uses: Busulfan is used to treat chronic myelogenous leukemia (CML). It does not cure the disease but helps to control it so that your quality of life is improved.
Busulphan Adverse reaction: Toxicity may include interstitial pulmonary fibrosis (“busulfan lung”), hyperpigmentation, seizures, hepatic (veno-occlusive disease) (VOD), emesis, and wasting syndrome.
Busulfan also induces thrombocytopenia, a condition of lowered blood platelet count and activity, and sometimes medullary aplasia.
3. Ehtylenediamine
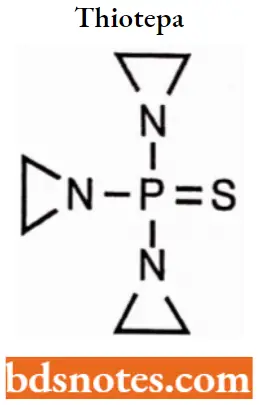
Thiotepa
“Can targeted interventions improve outcomes using antineoplastic medicinal chemistry knowledge? FAQs provided”
Thiotepa IUPAC name: 1,1′,1″-Phosphorothioyltriaziridine.
Thiotepa MOA: The alkyl group is attached to the guanine base of DNA, at the number 7 nitrogen atom of the imidazole ring. They stop tumor growth by crosslinking guanine nucleobases in DNA double-helix strands, directly attacking DNA.
This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. These drugs act nonspecifically.
Thiotepa SAR: Thiotepa containing the thiophosphoramide functionality was found to be more stable.
- When sulfur from thiotepa is replaced with oxygen the compound has instability which is named TEPA.
- Monoalkylation is also possible as a result of aziridine formation via hydrolysis of thiotepa.
Thiotepa Metabolism: Thiotepa is also metabolized by oxidative desulfurization mediated by CYP2B1 and CYP2C11.
Thiotepa Therapeutic Uses: Thiotepa is used in the palliation of many neoplastic diseases. The best results are found in the treatment of adenocarcinoma of the breast, adenocarcinoma of the ovary, papillary thyroid cancer, and bladder cancer.
Thiotepa Adverse reaction: The main side effect of thiotepa is bone marrow suppression resulting in leukopenia, thrombocytopenia, and anemia. Liver and lung toxicity may also occur.
4. Nitrosoureas
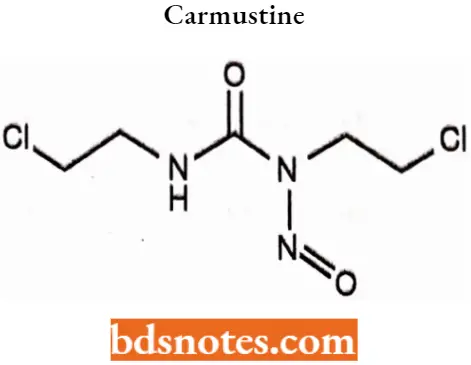
Carmustine
“Differential applications of cytotoxic vs targeted therapies: Notes explained”
Carmustine IUPAC name: l,3-Bis(2-chloroethyl)-1-nitrosourea.
Carmustine MOA: Acts as an alkylating agent, carmustine can form interstrand crosslinks in DNA, which prevents DNA replication and DNA transcription.
Carmustine SAR: Carmustine is an example of chloroethylnitrosoureas which is lipid soluble and can cross the blood-brain barrier.
Carmustine Metabolism: Hepatic and rapid with active metabolites. Metabolites may persist in the plasma for several days.
Carmustine Therapeutic Uses: Carmustine is used as an alkylating agent to treat several types of brain cancer including glioma, glioblastoma multiform, medulloblastoma, and astrocytoma), multiple myeloma, and lymphoma (Hodgkin’s and non-Hodgkin).
It is also used as part of a chemotherapeutic protocol in preparation for hematological stem cell transplantation, a type of bone marrow transplant.
Carmustine Adverse reaction: Bone marrow depression, pulmonary fibrosis, and effects on liver, kidneys, and eyes.
Antimetabolite
Antimetabolites can be used in cancer treatment, as they interfere with DNA production and therefore cell division and tumor growth.
- Because cancer cells spend more time dividing than other cells, inhibiting cell division harms tumor cells more than other cells.
- Antimetabolite drugs are commonly used to treat leukemia, cancers of the breast, ovary, and gastrointestinal tract, as well as other types of cancers.
- The main anti-metabolites are anti-folic acid, anti-purine, and anti-pyrimidine, acting on the cell S, and belong to the cell cycle-specific drugs.
As there is no significant difference between the tumor tissue and normal tissue metabolism process
1. Purine Antimetabolite
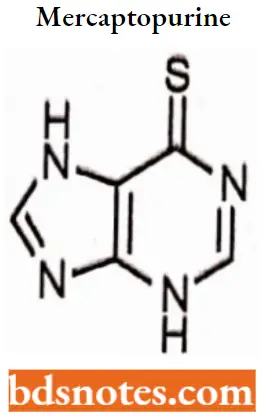
Mercaptopurine
“Steps to master antineoplastic medicinal chemistry for exams: Study plans vs mock tests: Notes guide”
Mercaptopurine IUPAC name: 3,7-dihydropurine-6-thione.
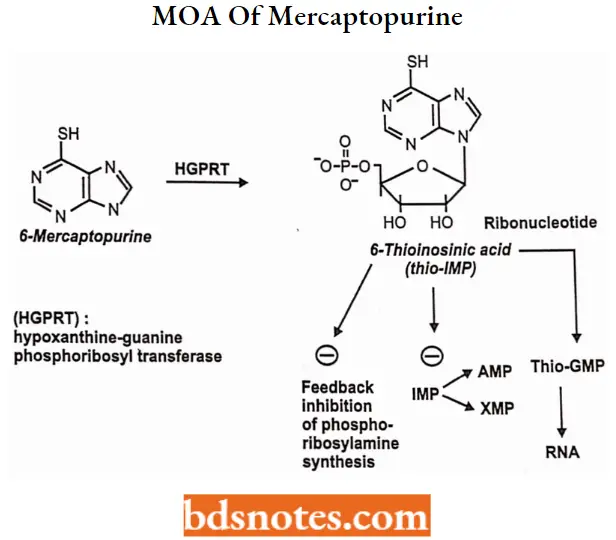
Mercaptopurine MOA: Mercaptopurine inhibits the conversion of inosine monophosphate to adenine and guanine nucleotides which are building blocks for RNA and DNA.
- Nucleotide formation: mercaptopurine converted into nucleotide analog, MP-ribose phosphate(6-thioinosinic acid, or TIMP)
- Inhibition of purine synthesis: TIMP can inhibit the first step of purine ring biosynthesis
- Incorporation into nucleic acids: TIMP is converted into thioguanine monophosphate (TGMP), which after phosphorylate to di and tri phosphates can be incorporated into RNA.
The deoxyribonucleotide analogs also formed are incorporated into DNA. This results in nonfunctional DNA and RNA.
“Role of diagrams in understanding antineoplastic structure-activity relationships: Questions answered”
Mercaptopurine SAR: The only compounds in this group that show significant tumor inhibitory effects are 6SCH3, 6-SCH2C6H6, and 6-SCH2C6H5N02(3′) purine.
- Compounds with CH3, Cl, OH, or SH at position 2 are inactive.
- S-benzyl derivatives were active.
- Heterocyclic derivatives of mercaptopurine such as azathioprine were designed to protect it from catabolic reactions but it is not significantly better than mercaptopurine.
Mercaptopurine Metabolism: Hepatic. Degradation primarily by xanthine oxidase. The catabolism of mercaptopurine and its metabolites is complex.
Mercaptopurine Therapeutic Uses: Mercaptopurine (6-MP) is a medication used for cancer and autoimmune diseases. Specifically, it is used to treat acute lymphocytic leukemia (ALL), chronic myeloid leukemia (CML), Crohn’s disease, and ulcerative colitis.
Mercaptopurine Adverse reaction: Some of the adverse reactions of taking mercaptopurine may include diarrhea, nausea, vomiting, loss of appetite, fatigue, stomach/abdominal pain, weakness, skin rash, darkening of the skin, and hair loss.
- Serious adverse reactions include mouth sores, fever, sore throat, easy bruising or bleeding, pinpointing red spots on the skin, yellowing of eyes or skin, dark urine, and painful or difficult urination.
- Other more serious side effects include black or tarry stools (melena), bloody stools, and bloody urine.
Thioguanine
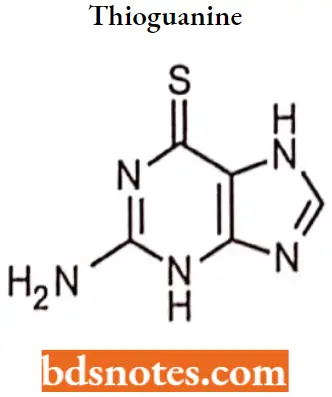
Thioguanine IUPAC name: 2-amino-1H-purine-6(7H)-thione
Thioguanine MOA: Thioguanine is a purine antagonist. It is a pro-drug that is converted intracellularly directly to thioguanine monophosphate (also called 6-thioguanylic acid) (TGMP) by the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRT).
- TGMP is further converted to the diand triphosphates, thioguanosinediphosphate (TGDP) and thioguanosine triphosphate (TGTP).
- The cytotoxic effect of thioguanine is a result of the incorporation of these nucleotides into DNA. Thioguanine has some immunosuppressive activity. Thioguanine is specific for the S phase of the cell cycle.
Thioguanine Metabolism: Hepatic. First converted to 6-thioguanilyic acid (TGMP). TGMP is further converted to the di-and tri-phosphates, thioguanosinediphosphate (TGDP) and thioguanosine triphosphate (TGTP) by the same enzymes that metabolize guanine nucleotides.
Thioguanine Therapeutic Uses: Acute leukemias in both adults and children, chronic myelogenous leukemia, inflammatory bowel disease, especially ulcerative colitis, psoriasis.
Thioguanine Adverse reactions: Nausea, vomiting, loss of appetite, and mouth sores may occur.
2. Pyrimidine antimetabolite
5-Fluorouracil
“How do case studies enhance comprehension of antineoplastic drug design? FAQ explained”
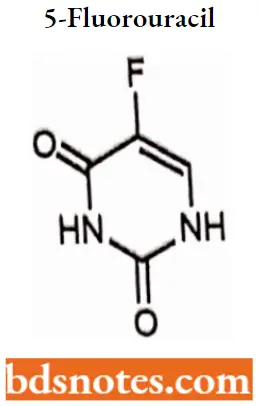
5-Fluorouracil IUPAC name: 5-Fluoro4H,3H-pyrlmldlne-2,4-dione.
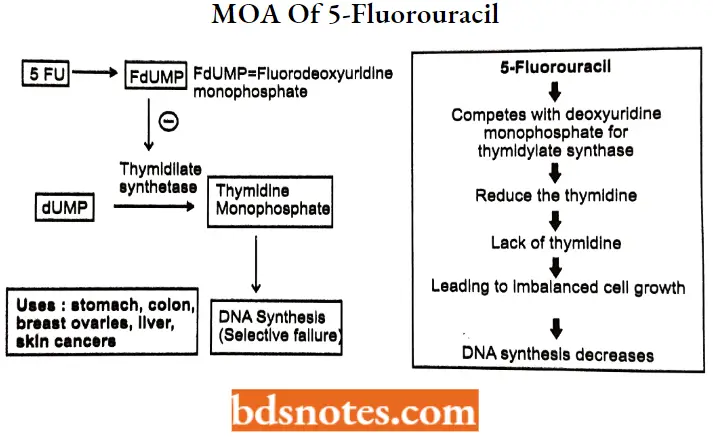
5-Fluorouracil MOA: 5-FU acts in several ways, but principally as a thymidylate synthase (TS) inhibitor.
Interrupting the action of this enzyme blocks the synthesis of the pyrimidine thymidine, which is a nucleoside required for DNA replication.
5-Fluorouracil SAR: Structure-activity relationship (SAR) analysis discovered that the anticancer activity was related to the configuration and that electron-withdrawing groups at 2-position or 4-position on the aryl group of arylsulfonyl derivatives of 5-fluorouracil could enhance the anticancer activity.
5 ethynyl uracil derivative of 5-FU improves the therapeutic index of 5-fluorouracil by 2 to 4 fold.
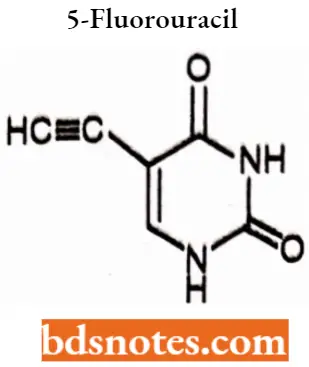
An atemative form of 5-FU is floxuridine which is a prodrug that is freely soluble in water.
5-Fluorouracil Metabolism: Hepatic. The catabolic metabolism of fluorouracil results in degradation products (For Example., C02, urea, and α-fluoro-β-alanine) which are Inactive.
5-Fluorouracil Therapeutic Uses: Fluorouracil has been given systemically for anal, breast, colorectal, oesophageal, stomach, pancreatic, and skin cancers (especially head and neck cancers).
It has also been given topically (on the skin) for actinic keratoses, skin cancers, and Bowen’s disease and as eye drops for the treatment of ocular surface squamous neoplasia.
5-Fluorouracil Adverse reactions: Adverse effects by frequency include Nausea, Vomiting, Diarrhea, Mucositis, Headache Myelosuppression, Alopecia (hair loss), Photosensitivity, Hand-foot syndrome, Maculopapular eruption, Itch, Cardiotoxicity, Persistent hiccups, Mood disorders.
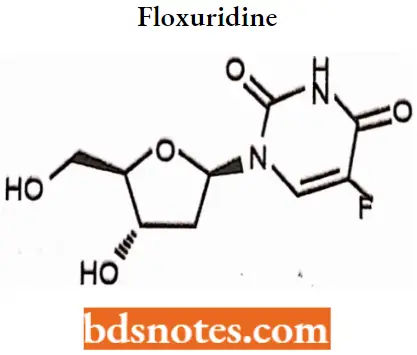
Floxuridine
“Early warning signs of poor performance in antineoplastic medicinal chemistry exams: Common questions”
Floxuridine IUPAC name: 5-Fluoro-1-[4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-lH-pyrimidine-2,4-dione.
Floxuridine MOA: Floxuridine is rapidly catabolized to 5-fluorouracil, which is the active form of the drug.
- The primary effect is interference with DNA synthesis and to a lesser extent, inhibition of RNA formation through the drug’s incorporation into RNA, thus leading to the production of fraudulent RNA.
- Floxuridine primarily works by stopping the growth of newly born cells. The drug essentially stops DNA from forming in new and rapidly developing cells, which is a sign of a cancerous cell.
- Therefore, the floxuridine kills the cancerous cells. Floxuridine is a pyrimidine analog that acts as an inhibitor of the S-phase of cells.
Floxuridine is a pyrimidine analog that acts as an inhibitor of the s-phase of cell division. This selectively kills rapidly dividing cells.
Floxuridine Metabolism: Metabolized in the liver by thymidine phosphorylase and Cytochrome P450 2A6 to 5-enzymes fluorouracil.
Floxuridine Therapeutic Uses: The drug is usually administered via an artery, and is most often used in the treatment of colorectal cancer.
- The quality of life and survival rates of individuals who receive continuous hepatic artery infusion of floxuridine for colorectal cancer metastases are significantly higher than control groups.
- Floxuridine can also be prescribed for the treatment of kidney and stomach cancers.
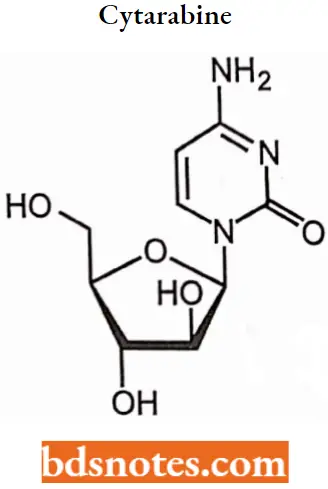
Cytarabine
“Asymptomatic vs symptomatic effects of outdated study methods: Answered”
Cytarabine IUPAC name: 4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5- (hydroxymethyl)oxolan-2-yl] pyrimidin- 2-one.
Cytarabine MOA: Cytarabine acts through direct DNA damage and incorporation into DNA. Cytarabine is cytotoxic to a wide variety of proliferating mammalian cells in culture.
- It exhibits cell phase specificity, primarily killing cells undergoing DNA synthesis (S-phase) and under certain conditions blocking the progression of cells from the G1 phase to the S-phase.
- Although the mechanism of action is not completely understood, it appears that cytarabine acts through the inhibition of DNA polymerase.
- A limited, but significant, incorporation of cytarabine into both DNA and RNA has also been reported.
Cytarabine SAR: The Sugar moiety of cytarabine is arabinose that has a 2′-OH group with β-configuration rather than the normal configuration which gives maximum activity.
Cytarabine is first converted into monophosphate and then its triphosphate derivative this change in configuration of the 2′ carbon results in a compound that has multiple activities.
Cytarabine Metabolism: Cytarabine is metabolized intracellularly into its active triphosphate form (cytosine arabinoside triphosphate).
Although the mechanism of action is not completely understood, it appears that cytarabine acts through the inhibition of DNA polymerase.
Cytarabine Therapeutic Uses: It is a chemotherapy medication used to treat acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), and non-Hodgkin’s lymphoma.
- It is given by injection into a vein, under the skin, or into the cerebrospinal fluid. Cytarabine also possesses antiviral activity, and it has been used for the treatment of generalized herpesvirus infection.
- However, cytarabine is not very selective in this setting and causes bone marrow suppression.
Cytarabine Adverse reactions: One of the unique toxicities of cytarabine is cerebellar toxicity when given in high doses, which may lead to ataxia.
Cytarabine may cause granulocytopenia and other impaired body defenses, which may lead to infection, and thrombocytopenia, which may lead to hemorrhage.
3. Folic acid Antagonist
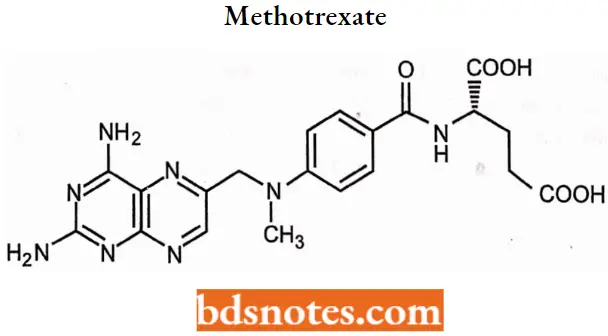
Methotrexate
“Can advanced tools supplement antineoplastic medicinal chemistry exam preparation? FAQs provided”
Methotrexate IUPAC name: (2S)-2-[(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzoyl)amino] pentanedioic acid.
Methotrexate MOA: Methotrexate competitively inhibits dihydrofolate reductase (DHFR), an enzyme that participates in tetrahydrofolate synthesis. The affinity of methotrexate for DHFR is about 1000-fold that of folate.
- DHFR catalyzes the conversion of dihydrofolate to the active tetrahydrofolate. Folic acid is needed for the de novo synthesis of the nucleoside thymidine, required for DNA synthesis.
- Also, folate is essential for purine and pyrimidine base biosynthesis, so synthesis will be inhibited. Methotrexate, therefore, inhibits the synthesis of DNA, RNA, thymidylates, and proteins.
Methotrexate SAR:
- Most structural variations such as alkylation of the amino group, partial reduction, and removal or relocation of heterocyclic nitrogens lead to decreased activity.
- Piritrexim and trimetrexate are analogs of methotrexate in which one or two nitrogens in the pyridine ring are replaced by carbons and the benzoylglutamicacid chain is replaced by a more lipophilic group which increases activity in vitro against some forms of methotrexate resistance.
Methotrexate Metabolism: Methotrexate undergoes hepatic and intracellular metabolism to polyglutamated forms which can be converted back to methotrexate by hydroxylase enzymes.
These polyglutamates act as inhibitors of dihydrofolate reductase and thymidylate synthetase.
Methotrexate Therapeutic Uses: Methotrexate was originally developed and continues to be used for chemotherapy, either alone or in combination with other agents.
- It is effective for the treatment of several cancers, including breast, head and neck, leukemia, lymphoma, lung, osteosarcoma, bladder, and trophoblastic neoplasms.
- It is used as a disease-modifying treatment for some autoimmune diseases, including rheumatoid arthritis, juvenile dermatomyositis, psoriasis, psoriatic arthritis, lupus, sarcoidosis, Crohn’s disease, eczema and many forms of vasculitis
Methotrexate Adverse reactions: The most common adverse effects include: hepatotoxicity (liver damage), ulcerative stomatitis, leukopenia and thus predisposition to infection, nausea, abdominal pain, fatigue, fever, dizziness, acute pneumonitis, rarely pulmonary fibrosis, and kidney failure.
Methotrexate is teratogenic and hence is not advised for either the prospective father to take it before or for the mother to take it before or during pregnancy and for a period after birth.
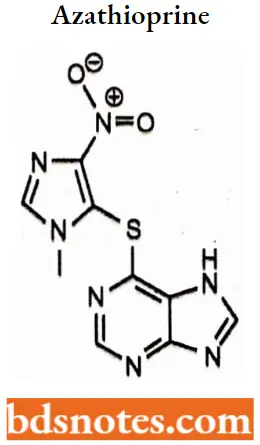
Azathioprine
Azathioprine is a purine analog with cytotoxic and immunosuppressive activity. Azathioprine is a prodrug that is converted by hepatic xanthine oxidase to its active metabolite 6-mercaptopurine (6-MP).
“Differential applications of traditional vs digital study resources: Q&A”
Azathioprine IUPAC name: 6-[(l-Methyl-4-nitro-lH-imidazol-5-yl)sulfanyl]-7H-purine.
Azathioprine MOA: Azathioprine inhibits purine synthesis, Purines are needed to produce DNA and RNA. By inhibiting purine synthesis, less DNA and RNA are produced for the synthesis of white blood cells, thus causing immunosuppression.
Azathioprine Metabolism: Primarily converted to the active metabolites 6-mercaptopurine and 6-thioinosinic acid via a nonenzymatic process and glutathione transferases.
Activation of 6-mercaptopurine occurs via hypoxanthine-guanine phosphoribosyltransferase (HGPRT) and a series of multi-enzymatic processes involving kinases to form 6-thioguanine nucleotides (6-TGNs) as major metabolites.
Azathioprine Therapeutic Uses: Azathioprine is used alone or in combination with other immunosuppressive therapy to prevent rejection following organ transplantation, and to treat an array of autoimmune diseases.
Including rheumatoid arthritis, pemphigus, systemic lupus erythematosus, Behqet’s disease, and other forms of vasculitis, Azathioprine acts to inhibit purine synthesis necessary for the proliferation of cells, especially leukocytes and lymphocytes,
Azathioprine Adverse reactions: Nausea and vomiting are common adverse effects, especially at the beginning of a treatment.
- Side effects that are probably hypersensitivity reactions include dizziness, diarrhea, fatigue, and skin rashes. Hair loss is often seen in transplant patients receiving the drug but rarely occurs under other indications.
- Because azathioprine suppresses the bone marrow, patients can develop anemia and will be more susceptible to infection; regular monitoring of the blood count is recommended during treatment.
Antineoplastic Antibiotics
An antineoplastic antibiotic is also known as an antidancer or antitumor antibiotic and it acts quite similar to quinolones.
- The main difference between antibiotics and antineoplastic antibiotics is the former acts on bacterial cells, while the latter acts on tumorous or cancerous cells in the human body.
- Antineoplastic antibiotic affects DNA synthesis and replication by inserting into DNA strands or by producing superoxide that causes breakage in DNA strands.
- And prevent the tumorous or cancerous cells from dividing further, Examples of antineoplastic antibiotics include doxorubicin, daunorubicin, bleomycin, mitomycin, and dactinomycin, all of which are derived from species of Streptomyces bacteria.
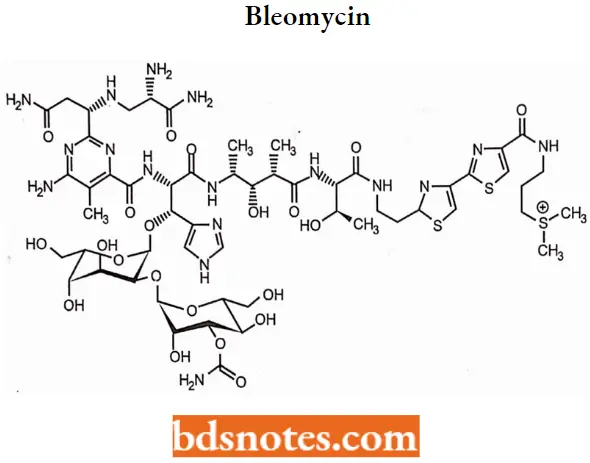
Bleomycin
Bleomycin is a natural product isolated from Streptomyces verticillus.
“Steps to incorporate AI into analyzing antineoplastic SAR: Questions and answers”
Bleomycin MOA: Bleomycin acts by induction of DNA strand breaks. Some studies suggest bleomycin also inhibits the incorporation of thymidine into DNA strands.
- DNA cleavage by bleomycin depends on oxygen and metal ions, at least in vitro.
- The exact mechanism of DNA strand scission is unresolved, but it has been suggested that bleomycin chelates metal ions (primarily iron), producing a pseudoenzyme that reacts with oxygen to produce superoxide and hydroxide free radicals that cleave DNA.
Bleomycin SAR:
- Bleomycin is inactivated by an intracellular enzyme named bleomycin hydrolase this structural change increases the pKa which results in poor binding to DNA.
- Racemization at the carbon atom substituted at the 2-position of the pyrimidine ring gives epi bleomycin which retains near about 25% of the antitumor activity of the parent bleomycin.
Bleomycin Metabolism: Bleomycin is metabolized primarily by the kidneys. Hence renal failure predisposes to impaired metabolism of the drug and increased toxicity.
Bleomycin Therapeutic Uses: Bleomycin is mostly used to treat cancer. This includes testicular cancer, ovarian cancer, Hodgkin’s disease, and less commonly non-Hodgkin’s disease.
Bleomycin is used intravenously in the palliative treatment of squamous cell head and neck cancers, and testicular and other genital carcinomas.
Bleomycin Adverse reactions: The most serious complication of bleomycin is pulmonary fibrosis and impaired lung function. It has been suggested that bleomycin induces sensitivity to oxygen toxicity.
Dactinomycin or Actinomycin D
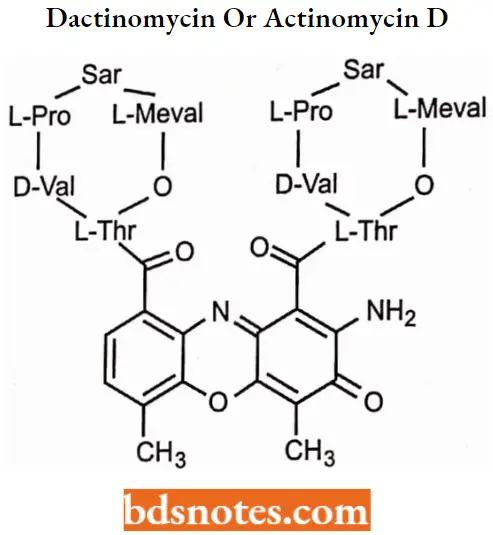
Dactinomycin MOA: This drug binds strongly, but reversibly, to DNA, interfering with the synthesis of RNA (prevention of RNA polymerase elongation) and, consequently, with protein synthesis.
Actinomycin D is shown to have the ability to inhibit transcription. Actinomycin D does this by binding DNA at the transcription initiation complex and preventing the elongation of the RNA chain by RNA polymerase.
Dactinomycin SAR: Changes in substituents on the actinomycin influence their binding to DNA, usually by making it less effective.
- Opening of lactone ring or changing the stereochemistry of an amino acid abolishes activity.
- Replacement of 4 and 6 methyl groups by other substituents reduces activity.
- Replacement of the 2-amino group also reduces activity.
Dactinomycin Metabolism: The drug’s 36-hour half-life is the result of a very high affinity for DNA, a large volume of distribution, and minimal metabolic breakdown.
Dactinomycin Therapeutic Uses: Dactinomycin is used for the treatment of various solid tumors and muscle-related cancers.
This includes Wilms tumor, rhabdomyosarcoma, Ewing’s sarcoma, trophoblastic neoplasm, testicular cancer, and certain types of ovarian cancer.
Dactinomycin Adverse reactions: Most people develop side effects. Common side effects include bone marrow suppression, vomiting, mouth ulcers, hair loss, liver problems, infections, and muscle pains.
Other serious side effects include future cancers, allergic reactions, and tissue death at the site of injection.
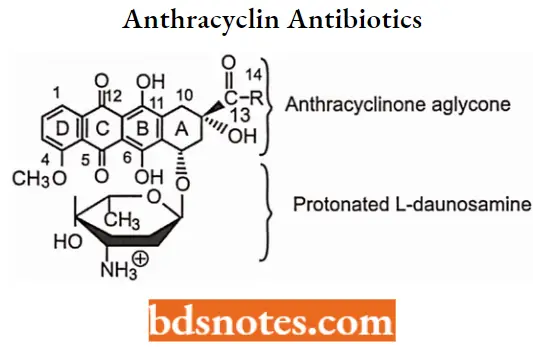
Anthracyclin Antibiotics
Anthracydine antineoplastic antibiotics are very closely related to the tetracycline antibacterials. Structurally, they are glycosides and contain a sugar portion (I-daunosamine) and a nonsugar organic portion.
- The nonsugar portion of glycosides is generically referred to as an aglycone. In anthracyclines, the aglycone moiety is specifically called anthracycline or anthraquinone.
- Anthracycline antibiotic structures are based on the anthracycline (anthraquinone) or (naphthoquinone) core with quinones, phenolic groups, and other substituents on the resonating ring structure A, B, and C and the saturated D ring.
“Role of digital tools in improving precision with antineoplastic drug tracking: FAQs explained”
Daunorubicin and doxorubicin were early chemotherapy agents in this class. Daunomycin (daunorubicin) was the first anthracycline compound to be characterized structurally and stereochemically.
- Daunorubicin is used in treating acute lymphoblastic and myeloblastic leukemias. Adriamycin (generic name doxorubicin) is similar to daunorubicin.
- Doxorubicin is one of the most widely used chemotherapeutic agents and is generally prescribed in combination with other drugs.
- Doxorubicin has a broad spectrum of activity. It is sometimes called “red devil chemo” partly due to its red color.
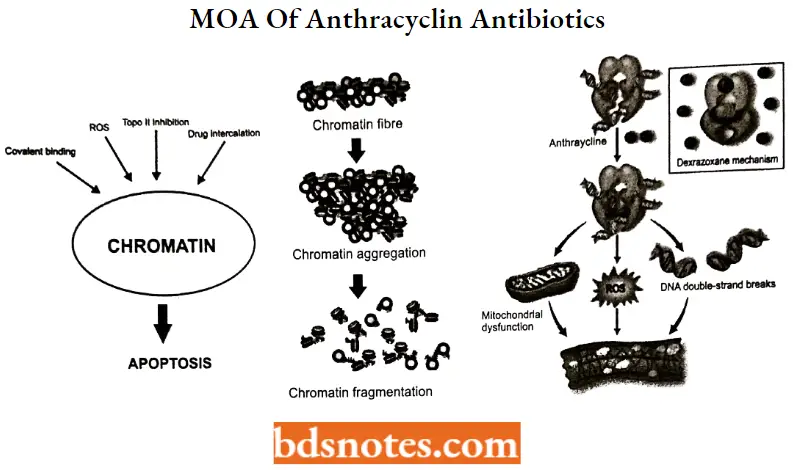
Anthracyclin Antibiotics MOA:
Anthracydines have four mechanisms of action:
- Inhibition of DNA and RNA synthesis by intercalating between base pairs of the DNA or RNA strand, thus preventing the replication of rapidly growing cancer cells.
- Inhibition of topoisomerase 2 enzymes, preventing the relaxing of supercoiled DNA and thus blocking DNA transcription and replication.
- Some sources say that topoisomerase 2 inhibitors prevent topoisomerase 2 from turning over which is needed for dissociation of topoisomerase 2 from its nucleic acid substrate.
- In other words, topoisomerase 2 inhibitors stabilize the topoisomerase 2 complex after it has broken the DNA chain. This leads to topoisomerase 2 mediated DNAdeavage, producing DNA breaks.
- Iron-mediated generation of free oxygen radicals that damage the DNA, proteins, and cell membranes.
- Induction of histone eviction from chromatin that deregulates DNA damage response, epigenome, and transcriptome.
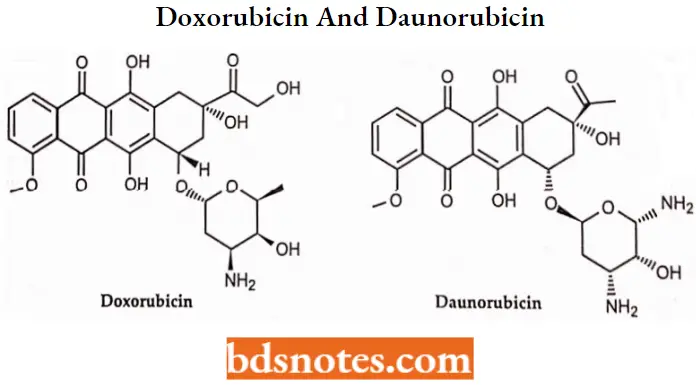
Anthracyclin Antibiotics SAR: Daunorubicin rapidly metabolized to Daunorubicinol by reducing its 13 keto group. This metabolite is 1/10th as active as daunorubicin.
- Many analogs of doxorubicin with changes in sugar moiety have been prepared for Example. Pirarubicinaccumalates more rapidly than doxorubicin in tumor cells and shows superior activity in animal models.
- Idarubicin one of the analogs of anthracycline antibiotic differs from daunorubicin by the lack of methoxy group at the 4-position and it appears to be less cardiotoxic than doxorubicin and daunorubicin.
- Chemically, valrubicin differs from its doxorubicin parent by the addition of a C14-valerate ester and a 3-trifluoroacetamide moiety. Both of these structural changes promote a more rapid and extensive penetration into tumor cells.
Anthracyclin Antibiotics Therapeutic Uses: Anthracyclines are used to treat various cancers and as of 2012 were among the most commonly used chemotherapeutic agents.
- Doxorubicin and its derivative, epirubicin, are used in breast cancer, childhood solid tumors, soft tissue sarcomas, and aggressive lymphomas.
- Daunorubicin is used to treat acute lymphoblastic or myeloblastic leukemias, and its derivative, idarubicin is used in multiple myeloma, non-Hodgkin’s lymphomas, and breast cancer.
Anthracyclin Antibiotics Adverse reactions: The organs and systems most affected by anthracyclines that lead to toxicities are
- Suppression of bone marrow regeneration,
- Suppression of gastrointestinal regeneration with concomitentmucositis and necrotizing colitis, and
- Myocardial toxicity upon total cumulative doses exceeding 300 mg/m2.
Plants Products as Antlneoplastlc Agents
The search for anti-cancer agents from plant sources started in earnest in the 1950s with the discovery and development of the vinca alkaloids, vinblastine, and vincristine, and the isolation of the cytotoxic podophyllotoxins.
As a result, the United States National Cancer Institute (NCI) initiated an extensive plant collection program in 1960, focused mainly on temperate regions.
1. Vinka Alkaloids
Vinca alkaloids belong to an important class of anti-cancer drugs. Vinka alkaloids include, including Vinblastine (VLB) and Vincristine (VCR), Vinorelbine (VRLB), and Vindesine (VDS) are obtained from the Madagascar periwinkle, Catharanthusroseus G. Don. (Apocynaceae).
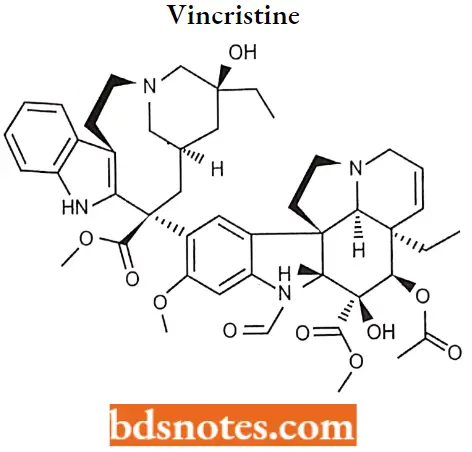
Vincristine
Vincristine (brand name, Oncovin), formally known as leurocristine, sometimes abbreviated “VCR”, is a vinca alkaloid from the Catharanthusroseus (Madagascar periwinkle), formerly Vincarosea and hence its name.
“How do advancements in technology enhance antineoplastic medicinal chemistry? Notes guide”
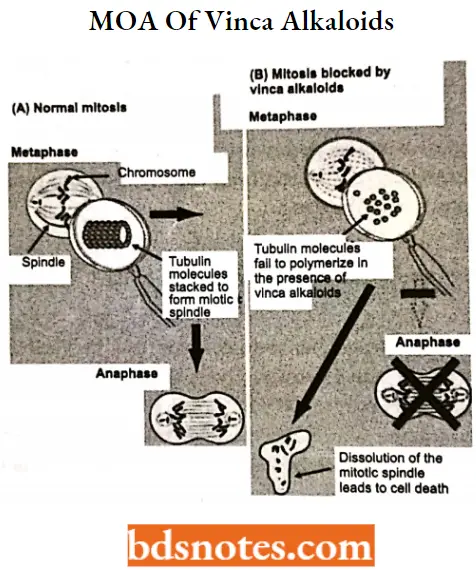
Vincristine MOA: Vincristine works partly by binding to the tubulin protein, stopping the tubulin dimers from polymerizing to form microtubules, causing the cell to be unable to separate its chromosomes during the metaphase.
- The cell then undergoes apoptosis. The vincristine molecule inhibits leukocyte production and maturation. A downside, however, to Vincristine is that it does not only affect the division of cancer cells.
- It affects all rapidly dividing cell types, making it necessary for the very specific administration of the drug.
Vincristine Metabolism: Vincristin metabolized by Cytochrome P450 isoenzymes of the CYP3A subfamily.
Vincristine Therapeutic Uses: Vincristine is delivered via intravenous infusion for use in various types of chemotherapy regimens.
- Its main uses are in non-Hodgkin’s lymphoma as part of the chemotherapy regimen CHOP, Hodgkin’s lymphoma as part of MOPP, COPP, BEACOPP, or the less popular Stanford V chemotherapy regimen.
- In acute lymphoblastic leukemia, and treatment for nephroblastoma (Wilms tumor, a kidney tumor most common in young children). It is used in combination with prednisone to treat childhood leukemia.
Vincristine Adverse reactions: The main side-effects of vincristine are peripheral neuropathy, hyponatremia, constipation, and hair loss.
Vinblastine:
Vinblastine (VLB) is a major naturally occurring active compound. Vinblastine sulfate is the salt of an alkaloid extracted from Vincarosea Linn., a common flowering herb known as the periwinkle (more properly known as Catharanthusroseus G. Don).
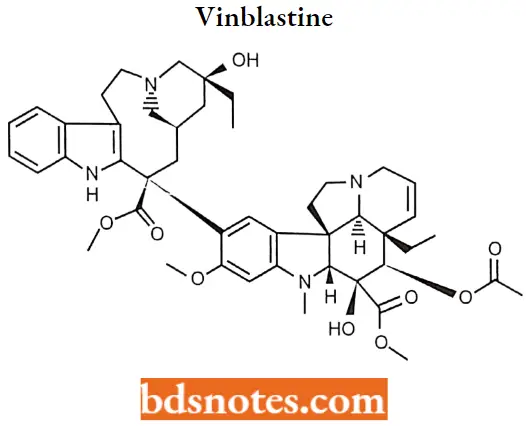
Vinblastine MOA: The antitumor activity of vinblastine is thought to be due primarily to the inhibition of mitosis at metaphase through its interaction with tubulin.
Vinblastine binds to the microtubular proteins of the mitotic spindle, leading to crystallization of the microtubule and mitotic arrest or cell death.
“Early warning signs of outdated methods in antineoplastic studies: Common questions”
Vinblastine SAR
- The deacetyl derivative of vinblastine is more active than the parent compound.
- The tertiary amino group of vinblastine is highly essential for the formation of salt which is freely soluble in water.
Vinblastine Metabolism: Metabolism of vinblastine is mediated by hepatic cytochrome P450 3A isoenzymes.
Vinblastine Therapeutic Uses: Vinblastine is an anti-cancer medication prescribed for various cancers such as Hodgkin’s lymphoma, non-Hodgkins lymphoma, breast cancer, testicular cancer, mycosis fungoides, Kaposi’s sarcoma related to acquired immunodeficiency syndrome (AIDS), Letterer-Siwe disease.
Vinblastine is also used to treat non-small cell lung cancer, bladder cancer, head and neck cancer, cervical cancer, idiopathic thrombocytopenia purpura, and autoimmune hemolytic anemia.
Adverse reactions: Vinblastine includes adverse effects are nausea and vomiting which usually lasts less than 24 hours, stomach pain, constipation, diarrhea, jaw pain, headache, Or other ache, thinned or brittle hair, exposed areas of the skin may become easily sunburned.
2. Epipodophylloloxin
The most studied lignan, podophyllotoxin, and its semisynthetic derivatives (etoposide, teniposide, etoposide phosphate), are particularly interesting at a curative level due to their cytotoxic properties. These semi-synthetic derivatives are used in chemotherapy for lung cancer.
Etoposide
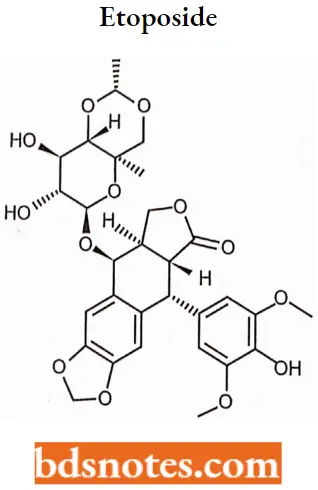
Etoposide Is a semisynthetic derivative of podophyllotoxin from the rhizome of the wild mandrake (Podophylhnnpcllatum), More specifically, it is a glycoside of podophyllotoxin with a Dglucose derivative.
“Asymptomatic vs symptomatic effects of ignoring new trends in antineoplastic research: Answered”
Etoposide MOA: Etoposide inhibits DNA topoisomerase 2, thereby inhibiting DNA re-ligation. This causes critical errors in DNA synthesis at the premitotic stage of cell division and can lead to apoptosis of the cancer cell.
- Etoposide is cell cycle-dependent and phase-specific, affecting mainly the S and G2 phases of cell division. Inhibition of the topoisomerase 2 alpha isoform results in the anti-tumor activity of etoposide.
- The drug is also capable of inhibiting the beta isoform but inhibition of this target is not associated with the anti-tumor activity.
Etoposide SAR: There is a difference between the structure of the originally isolated natural products and semisynthetic derivatives which involves stereochemical relation between the substituents at the 1,4 position.
- Podophyllotoxins have cis-stereochemistry while active drugs possess trans-configuration.
- A variety of functional groups are present in the structure of etoposide including lactone, phenol, aromatic ether, acetal, and glucopyranoside groups, None of these groups form stable salt which is useful for solubilizing etoposide.
- Teniposide is chemically similar to Etoposide being distinguished only by a methyl rest where teniposide has a thienyl which is highly lipophilic showing a water or octanol partition coefficient of approx. 100.
Etoposide Metabolism: Primarily hepatic (through O-demethylation via the CYP450 3A4 isoenzyme pathway) with 407c excreted unchanged in the urine.
Etoposide also undergoes glutathione and glucuronide conjugation. Prostaglandin synthases are also responsible for the conversion of etoposide to Odemethylated metabolites (quinone).
Etoposide Therapeutic Uses: Etoposide is used as a form of chemotherapy for cancers such as Kaposi’s sarcoma, Ewing’s sarcoma, lung cancer, testicular cancer, lymphoma, nonlymphocytic leukemia, and glioblastomamultiforme.
It is also sometimes used in a conditioning regimen before a bone marrow or blood stem cell transplant.
Etoposide Adverse reactions: Adverse effects of etoposide include low blood pressure, hair loss, pain and or burning at the 4 sites, constipation or diarrhea, and metallic food taste.
- Bone marrow suppression leads to decreased white blood cell counts (leading to increased susceptibility to infections), and low red blood cell counts (anemia).
- Low platelet counts (leading to easy bruising and bleeding), nausea and vomiting, allergic-type reactions, rash, and fever often occur shortly after 4 administration.
Miscellaneous
Cisplatin
Cisplatin was discovered in 1845 and licensed for medical use in 1978/1979. It is on the World Health Organization’s List of Essential Medicines, the most effective and safe medicines needed in a health system.
“Differential applications of traditional vs cutting-edge antineoplastic techniques: Notes explained”
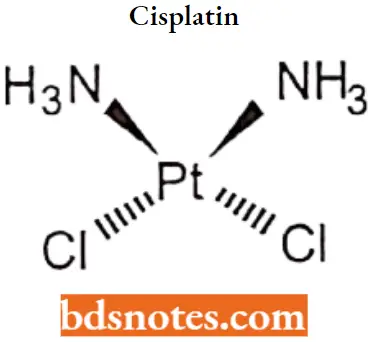
Cisplatin IUPAC name: (SP-4-2)-diamminedichloroplatinum (2)
Cisplatin MOA: Cisplatin interferes with DNA replication, which kills the fastest proliferating cells, which in theory are carcinogenic.
- Following administration, one chloride ion is slowly displaced by water to give the aqua complex ris-[PtCl(NH3)2(H20)]t+, in a process termed aquation.
- Cisplatin crosslinks DNA in several different ways, interfering with cell division by mitosis. The damaged DNA elicits DNA repair mechanisms, which in turn activate apoptosis when repair proves impossible.
- Although cisplatin is frequently designated as an alkylating agent, it has no alkyl group and it therefore cannot carry out alkylating reactions. It is correctly classified as alkylating-like.
Cisplatin SAR:
- Cisplatin is a platinum complex containing two ammonia molecules and two chlorine atoms in cis- a configuration that gives maximum activity.
- Transform rapidly deactivated.
- It is a ds-isomer in which one pair of ligands are monodentate anions of intermediate leaving ability (such as chlorine) and the other pair are mono-or bidentate amines which is essential for antitumor activity.
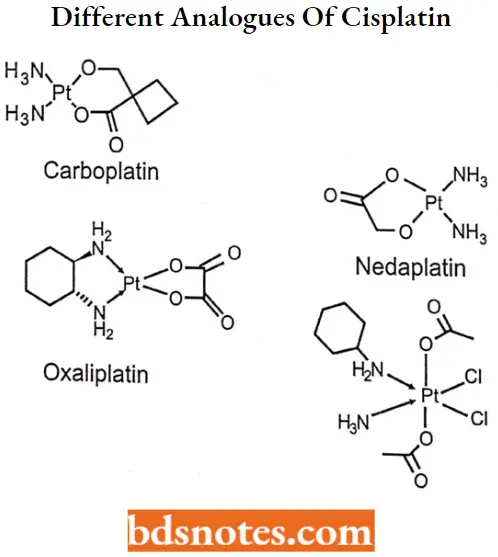
- Different analogs of cisplatin are available as follows.
“Can bioinformatics revolutionize antineoplastic drug design? FAQs provided”
Cisplatin Metabolism: Metabolism of cisplatin to nephrotoxin tubule cells. This activation begins with the formation of a glutathione-conjugate that is metabolized to a cysteinyl-glycine-conjugate, to a cysteine-conjugate, and finally to a reactive thiol.
Cisplatin Therapeutic Uses: Cisplatin is administered intravenously as a short-term infusion in normal saline for the treatment of solid malignancies.
- It is used to treat various types of cancers, including sarcomas, and some carcinomas (For Example., small cell lung cancer, squamous cell carcinoma of the head and neck, and ovarian cancer).
- Lymphomas, bladder cancer, cervical cancer, and germ cell tumors. Cisplatin is particularly effective against testicular cancer; the cure rate was improved from 10% to 85%.
Cisplatin Adverse reactions: Nausea, vomiting, loss of appetite, diarrhea, and loss of taste may occur. Nausea and vomiting can be quite severe and persistent.
Drug therapy is used to prevent or relieve nausea and vomiting. Temporary hair loss may occur. Neurotoxicity (nerve damage), ototoxicity, nephrotoxicity etc.
Mitotane
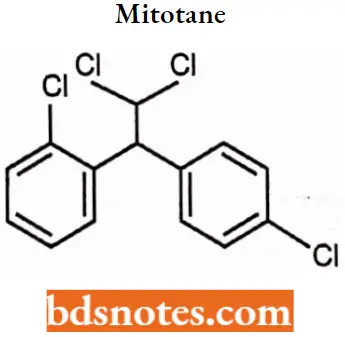
Mitotane IUPAC name: (RS)-1-chloro-2-[2,2-dichloro-1-(4-chlorophenyl)-ethyl]-benzene.
Mitotane MOA: Mitotane is an oral chemotherapeutic agent indicated in the treatment of inoperable adrenal cortical carcinoma of both functional and nonfunctional types. Mitotane can best be described as an adrenal cytotoxic agent, although it can cause adrenal inhibition, apparently without cellular destruction.
Mitotane Metabolism: About 65% of the ingested drugs were found to pass in the stool. The drug appeared in the urine in metabolized forms: mono- and dihydroxylated derivatives of mitotane. These latters found as well in the stools.
Mitotane Therapeutic Uses: Mitotane is a unique antineoplastic agent used solely in the therapy of metastatic, unresectable adrenocortical carcinoma.
Mitotane has been associated with a high rate of serum enzyme elevation during therapy, but has had limited clinical use and has not been linked to instances of clinically apparent acute liver injury.
Mitotane Adverse Reactions: The use of mitotane is unfortunately limited by side effects. Includes, anorexia and nausea (88%), diarrhea (38%), vomiting (23%), decreased memory and ability to concentrate (50%), rash(23%), gynecomastia (50%), arthralgia (19%), and leukopenia (7%).
Synthesis of drugs
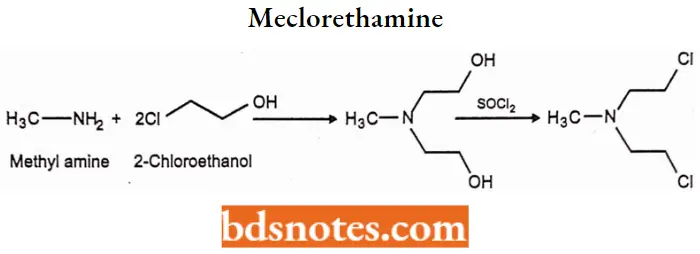
Meclorethamine
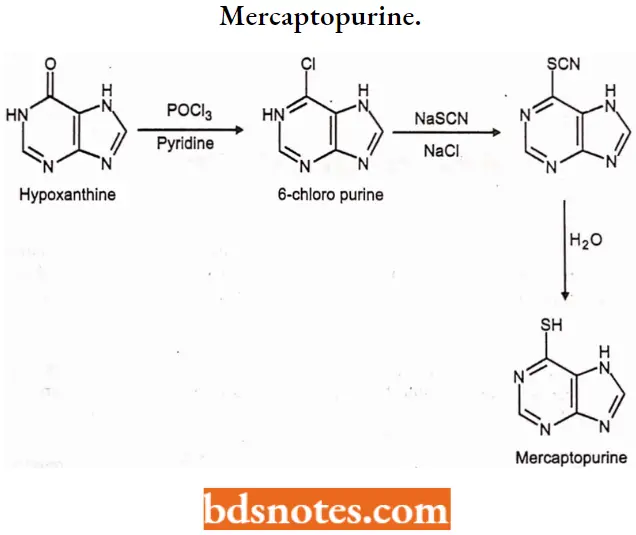
1. Mercaptopurine
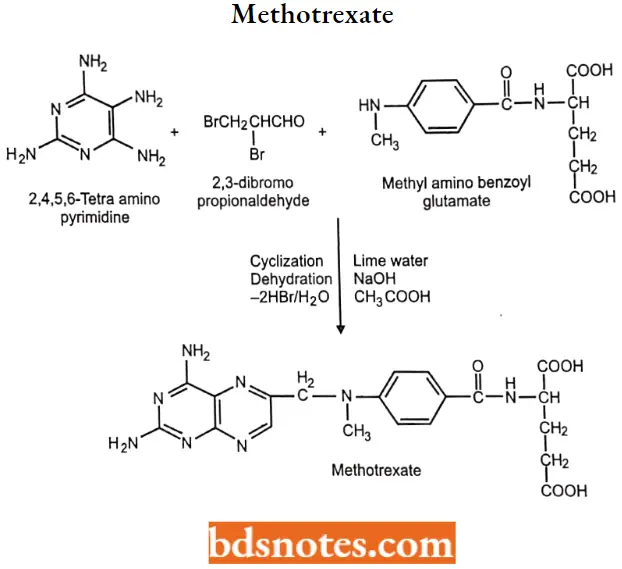
2. Methotrexate
Antineoplastic Drugs Multiple Choice Questions And Answers
Question 1. What term is used to indicate the ability of a cancer to invade other parts of the body and to produce secondary tumors?
- Carcinogenesis
- Apoptosis
- Metastasis
- Mutagen
Answer: 3. Metastasis
Question 2. The following anticancer agent is called busulfan.
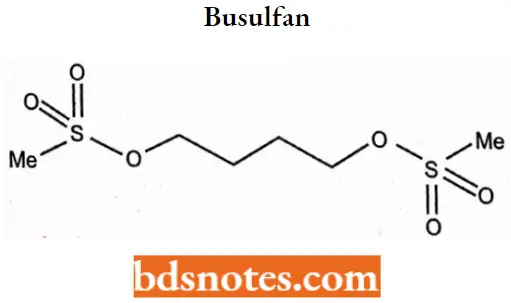
What is the drug’s mode of action?
- Metalizing agent
- Intercalator
- Chain terminator
- Alkylating agent
Answer: 4. Alkylating agent
Question 3. The following agent is used for the treatment of lenticular and ovarian cancers, Which of the following statements IN untrue regarding the above structure?
- The chlorine groups arc displaced before the drug becomes active
- Intrastrand cross-linking of DNA results from the action of the agent
- Base pairing between adenine and thymine is disrupted by the agent
- The compound acts as a metalling agent
Answer: 3. Base pairing between adenine and thymine is disrupted by the agent
Question 4. Among anticancer antibiotics: most toxic_________
- Plicamycin (Mithramycin)
- Dactinomycin (Cosmcgen)
- Doxorubicin (Adriamycin)
- Bleomycin (Blenoxane)
Answer: 3. Doxorubicin (Adriamycin)
Question 5. The most useful alkylating drug currently available:
- Melphalan (Alkeran)
- Cyclophosphamide (Cytoxan)
- Lomustine (CCNU,CeeNU)
- Thiopeta (Thioplex)
Answer: 2. Cyclophosphamide (Cytoxan)
Question 6. One of the following drugs binds with tubulin and arrests the cell cycle In metaphase.
- Vinka
- Nitrogen mustards
- Antimetabolites
- Alkylating agent
Answer: Vinka
Question 7. Methotrexate inhibits.
- Glutathione reductase
- Alanine transferase
- Dihydrofolate Reductase
- Adenine deaminase
Answer: 3. Dihydrofolate Reductase
Question 8. All are alkylating agents except.
- Busulphan
- Cisplatin
- Procarbazine
- Methotrexate
Answer: 4. Methotrexate
Question 9. Which drugs inhibit RNA and DNA synthesis?
- Carmustine
- Paclitaxel
- Doxorubicin
- Interferon alpha
Answer: 3. Doxorubicin
Question 10. Which drug is an extract from the “Pcriwhinkle plant”?
- Busulfan
- Chlorambucil
- Lomustine
- Vincristine
Answer: 4. Vincristine
Antineoplastic Drugs Short Questions And Answers
Question 1. How cancer spread?
Answer:
Cancer is the rapid creation of abnormal cells that grow beyond their usual boundaries, and which can then invade adjoining parts of the body and spread to other organs.
This process is referred to as metastasis. metastases are the major cause of death from cancer.
Question 2. What is the difference between a benign tumor and a malignant tumor?
Answer:
A benign tumor is not a cancerous tumor. Unlike cancer tumors, a non-cancerous tumor is unable to spread throughout the body.
- A non-malignant tumor can be serious if they are pressing a primary nerve,- a main artery, or compresses brain matter.
- Malignant Tumors can multiply uncontrollably, metastasize (spread) to various parts of the body, and invade surrounding tissue.
Question 3. Explain the cell cycle in cancer cells.
Answer:
The cell cycle, the process by which cells progress and divide, lies at the heart of cancer.
- The cell cycle involves a complex series of molecular and biochemical signaling pathways. The cell cycle has four phases:
- The Gl, or gap, phase, in which the cell grows and prepares to synthesize DNA.
- The S, or synthesis, phase, in which the cell synthesizes DNA.
- The G2, or second gap, phase, in which the cell prepares to divide.
- The M, or mitosis, phase, in which cell division occurs.
Question 4. Explain the MOA of Cyclophosphamide.
Answer:
The main effect of cyclophosphamide is due to its metabolite phosphoramide mustard. This metabolite is only formed in cells that have low levels of ALDH.
Phosphoramide mustard forms DNA crosslinks both between and within DNA strands at guanine N-7 positions (known as interstrand and intrastrand cross-linkages, respectively). This is irreversible and leads to cell apoptosis.
Question 5. Give SAR of mercaptopurine.
Answer:
- The only compounds in this group that show significant tumor inhibitory effects are 6SCH3, 6- SCH2C6H6, and b-SCH2C6H5NO2 (3’) purine.
- Compounds with CH3, Cl, OH, or SH at position 2 are inactive.
- S-benzyl derivatives were active.
- Heterocyclic derivatives of mercaptopurine such as azathioprine were designed to protect it from catabolic reactions but it is not significantly better than mercaptopurine.
Leave a Reply