Carbohydrates Biochemistry Essay Question And Answers
Define carbohydrates. Classify them and add a note on the biomedical importance of carbohydrates.
(Or)
Classification of carbohydrates
(or)
Describe the aerobic glycolysis in muscle and compute energy.
(or)
Outline the process of glycolysis. Define substrate-level phosphorylation. Give two examples.
Carbohydrates biochemistry essay questions and answers
“Understanding carbohydrates biochemistry through FAQs: Structure, functions, and uses explained”
“Importance of studying carbohydrates biochemistry for medical students: Questions explained”
Answer:
Definition:
- Carbohydrates may be defined as polyhydroxy aldehydes or ketones or compounds which produce them on hydrolysis.
Read And Learn More: BDS Previous Examination Question And Answers
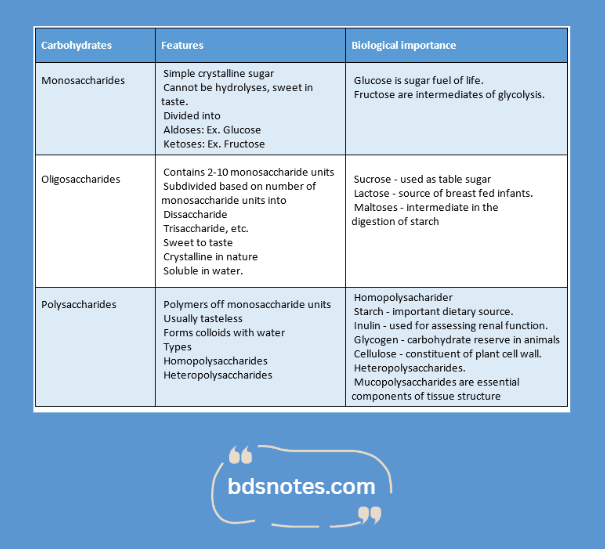
Classification:
- Carbohydrates are broadly classified based on the number of sugar units.
- They are:
“Common challenges in mastering carbohydrates biochemistry notes effectively: FAQs provided”
“Factors influencing success with carbohydrates biochemistry essays: Q&A”
Read And Learn More: BDS Previous Examination Question And Answers
Leave a Reply